Figure 8. Knockdown of STAT3 attenuates EGFR-mediated apoptosis in MDA-MB-468 cells.
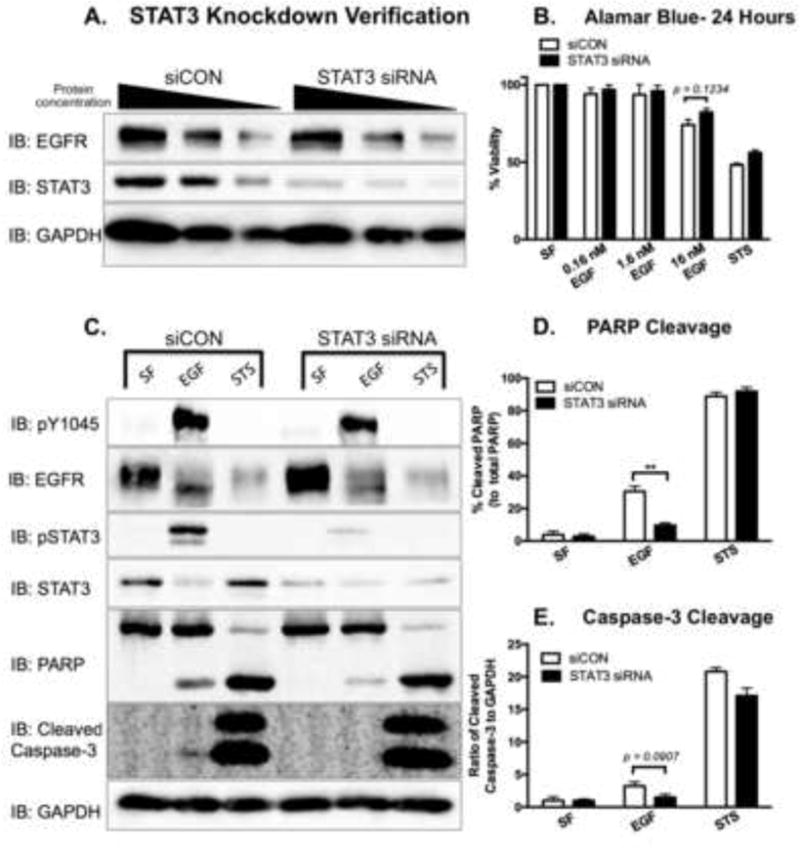
A. MDA-MB-468 cells were transfected with either 200 nM control siRNA (siCON) or 200 nM STAT3 siRNA for 72 hours. Cell lysates were prepared, and decreasing protein concentrations (40, 20, and 10 μg) were resolved by 10% SDS PAGE and immunoblotted for EGFR, STAT3, and GAPDH. B. Fourty-eight hours post-transfection, siCON and STAT3 siRNA-transfected cells were treated for 24 hours with 0 (SF), 0.16, 1.6, 16 nM EGF or 168 nM Staurosporine (STS) as a positive control. Cell viability was assessed by an Alamar Blue assay. Data are expressed as the average ± SEM (n=3). C. Cleaved PARP and cleaved Caspase-3 were assessed in siCON and STAT3 siRNA transfected cells. Seventy-two hours post-transfection, cells were coincidently exposed to SF DMEM and 16 nM EGF for 24 hours or 1 μM STS for 4 hours prior to harvesting. Cell lysates (40 μg) were resolved on either a 10% SDS PAGE (pY1045, PARP, and GAPDH) or a 15% SDS PAGE (pSTAT3 and cleaved Caspase-3), and were assessed for the indicated proteins via immunoblot analysis. Densitometry quantification of western blot data of cleaved PARP (D.) and cleaved Caspase-3 (E.). The cleaved PARP band intensities were normalized to and plotted as a percentage of total PARP. The intensity of cleaved Caspase-3 was normalized to the levels of GAPDH. Data are expressed as the average ± SEM (n=3).
