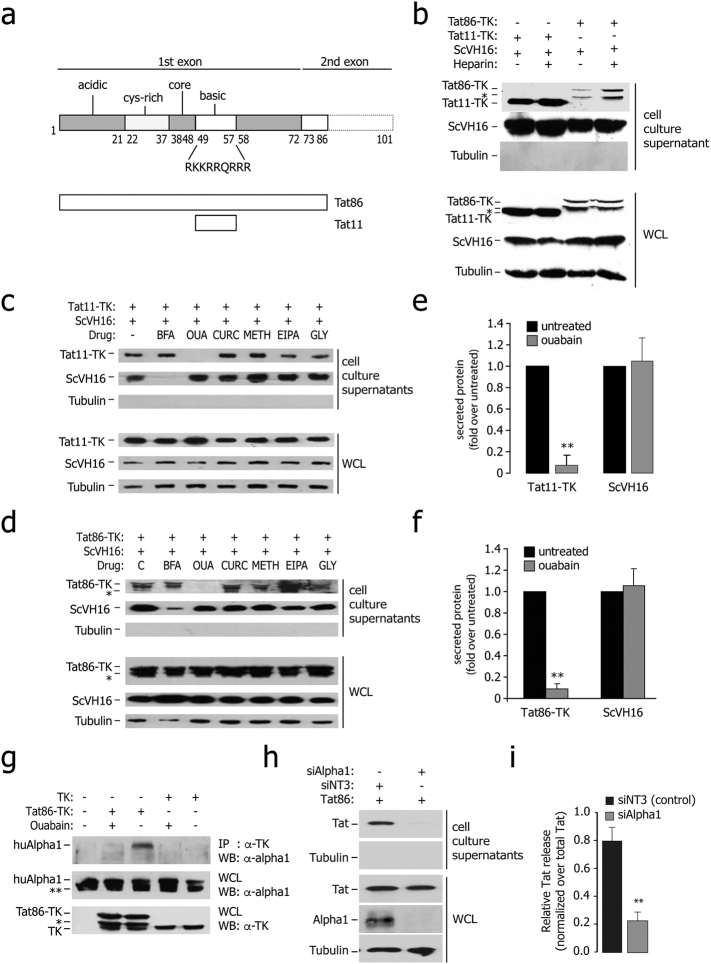
Fig. 1.
Ouabain-sensitive secretion of Tat from the expressing cells.
(a) Schematic representation of the major functional domains of HIV-1 Tat (acidic, cysteine-rich, core, and basic). Tat has 101 aa in several clinical isolates and 86 aa in the laboratory strain HX2B. The amino acidic sequence of the basic domain of the protein, which imparts the protein intercellular trafficking capability, is indicated. The lower part of the panel shows a schematic representation of the two Tat proteins used in this study (Tat11, corresponding to the Tat basic domain plus two additional amino acids at both extremities, and Tat86).
(b) Tat86-TK and Tat11-TK are released from the expressing cells and bind extracellular HSPG upon secretion. The immunoblots in the upper panel show the amount of proteins released in the cell culture supernatants of cells transfected with Tat86-TK, Tat11-TK or scVH16-SV5, treated or untreated with 25 μM soluble heparin. The immunoblots in the lower part show the levels of intracellular protein expression in the same samples. WCL: whole cell lysates. The asterisk (*) indicates an additional band present in the Tat86-TK immunoblots, probably corresponding to a degradation product. Lack of tubulin immunoreactivity in the supernatants indicates the absence of appreciable cell lysis.
(c) Sensitivity of Tat11-TK and scFv secretion to the indicated drugs. HEK293T cells were co-transfected with Tat and scFV expressing plasmids and treated with the indicated metabolic drugs. The amount of secreted protein was assessed by western blot on cell culture supernatants, while protein expression and loading was checked on whole cell lysates (WCL). BFA: brefeldin A (10 μM); OUA: ouabain (25 μM); CURC: curcumin (50 μM); METH: methylamine (1 mM); EIPA: 5-(N-ethyl-N-isopropyl)amiloride (20 μM); GLY: glyburide (10 μM).
(d) Sensitivity of Tat86-TK and scFv secretion to the indicated drugs. HEK293T cells were co-transfected with Tat and scFV expressing plasmids and treated with the indicated metabolic drugs. The amount of secreted protein was assessed by western blot on cell culture supernatants, while protein expression and loading was checked on whole cell lysates (WCL). BFA: brefeldin A; OUA: ouabain; CURC: curcumin; METH: methylamine; EIPA: 5-(N-ethyl-N-isopropyl)amiloride; GLY: glyburide.
(e) Quantification of Tat11-TK and ScVH16 secretion in ouabain-treated cells. The amount of extracellular proteins, normalized over the levels of intracellular expression, was assessed after a 4 h incubation. Data are mean ± sem of three independent experiments. **P-value < 0.01.
(f) Quantification of Tat86-TK and ScVH16 secretion in ouabain-treated cells. The amount of extracellular proteins, normalized over the levels of intracellular expression, was assessed after a 4 h incubation. Data are mean ± sem of three independent experiments. **P-value < 0.01.
(g) Tat co-immunoprecipitates with the endogenous Na+,K+-ATPase α1 subunit; binding is sensitive to ouabain. HEK293T cells were transfected with Tat86-TK or TK as a control and treated with ouabain (25 μM) as indicated. The antibodies used for immunoprecipitation and subsequent western blots are indicated on the right side. Expression of endogenous α1 and either of the transfected proteins was verified in whole cell lysates (WCL). The bands marked with (*) are degradation products.
(h) Downregulation of cellular Na+,K+-ATPase α1 subunit impairs Tat release. The amount of released Tat was monitored in cell culture supernatants of Na+,K+-ATPase α1-knock down cells by immuno blotting with an anti-Tat antibody; protein expression and loading were checked in whole cell lysates (WCL).
(i) Quantification of the levels of Tat secretion after α1 RNAi knock down. Data are mean ± sem of three independent experiments.