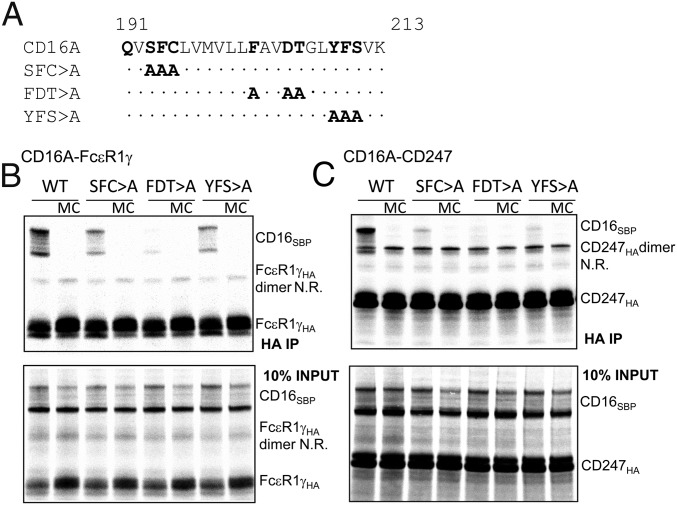
Fig. 2.
The effects of CD16A triple block mutants on association with CD247 or FcεR1γ. Three triple mutants targeting groups of polar and aromatic residues along the length of the CD16A transmembrane domain were prepared (A), and the ability of these mutants to assemble with adaptor modules, FcεR1γ or CD247, was evaluated (B and C). After immunoprecipitation using anti-HA antibody, complex formation with FcεR1γ (B) and CD247 (C) was analyzed in 12% SDS/PAGE gels in reducing conditions. Then 10% of assembly reactions without immunoprecipitation were analyzed in parallel as loading controls. As can be observed in the gels, dimers of FcεR1γ, and especially CD247, were not completely reduced in these conditions. MC, mixing control in which CD16A and FcεR1γ/CD247 were translated in separate reactions and mixed just before detergent extraction; N.R., nonreduced.