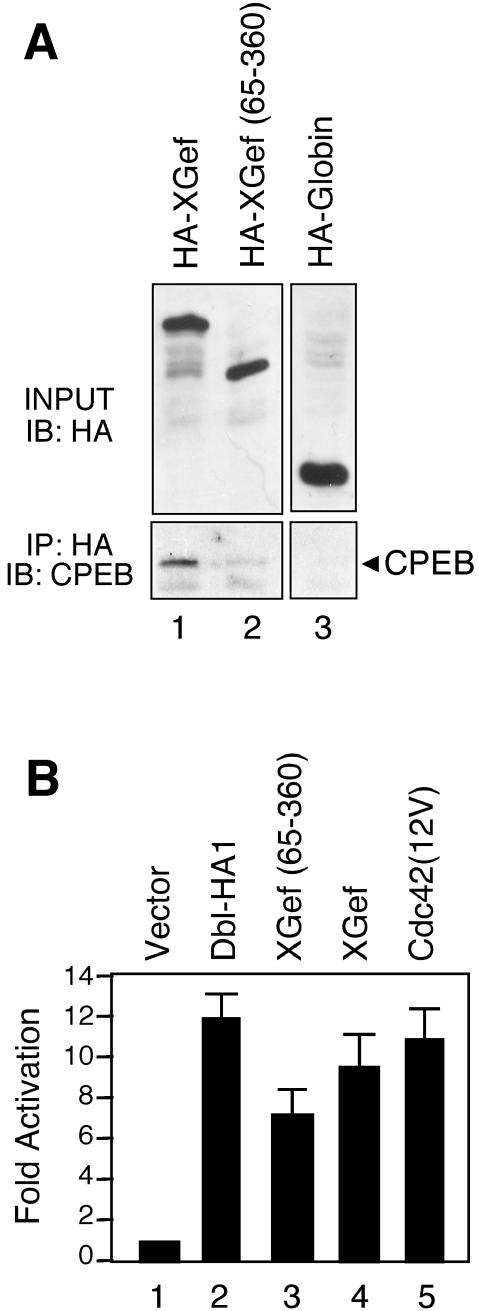
Figure 5.
XGef(65–360) has very reduced binding to endogenous CPEB and retains exchange factor activity. (A) Protein complexes from extracts prepared from oocytes overexpressing HA-XGef, HA-XGef(65–360), or HA-Globin were immunoprecipitated with anti-HA antibody. Input (INPUT) and protein G-agarose–bound (IP: HA) samples were analyzed by SDS-PAGE and immunoblotting with anti-HA (IB: HA) and anti-CPEB (IB: CPEB) antibodies, respectively. (B) Activation of (SREM)2-driven luciferase expression by XGef(65–360). NIH 3T3 cells were transiently transfected with 2.5 μg of the indicated constructs along with the reporter construct (SREm)2-luciferase (2.5 μg) and pCMVnlac (0.5 μg) as an internal control for transfection efficiency and growth inhibition. Luciferase and β-galactosidase activity were measured and expressed as fold activation relative to the level of activation seen with the vector control. Luciferase activity was then standardized relative to the β-galactosidase activity. Data shown are representative of three independent experiments performed on triplicate plates.