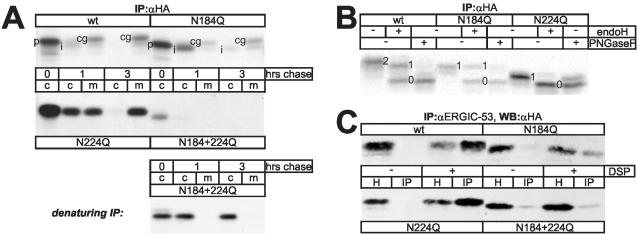
Figure 2.
Characterization of procatZ N-glycosylation-site mutants. (A) Top: CHO cells were transfected with wt or glycosylation-site mutant procatZ cDNAs, pulsed for 5 min with [35S]methionine, and chased for the indicated times. Intracellular and secreted procatZ was recovered by anti-HA immunoprecipitation (fluorogram). c, cells; m, medium; p, procatZ; i, intermediate catZ; cg; complex glycosylated catZ. Bottom: pulse-chase analysis as in top panel but using denaturing immunoprecipitation. Note that under these experimental conditions N184 + 224Q is readily detectable. (B) ProcatZ protein secreted from transfected CHO cells after a 5-min pulse and a 3-h chase was isolated by anti-HA immunoprecipitation and subjected to endoH or PNGase F digestion (fluorogram). Numbers indicate doubly-, singly-, and nonglycosylated procatZ. Wt-procatZ and N184Q are partially resistant to endoH, whereas N224Q is almost completely endoH-sensitive. (C) Cross-linking and coimmunoprecipitation of GMAA-ERGIC-53 and procatZ mutants. Anti-ERGIC-53 immunoprecipitates from GMAA-cells expressing the indicated procatZ mutants and treated with or without DSP were analyzed for the presence of procatZ by anti-HA immunoblotting. One percent of the total homogenate (H) was loaded as an expression control. Note that DSP-dependent coisolation is decreased by N184Q, but unaffected by N224Q. IP, immunoprecipitation; WB, Western blotting.