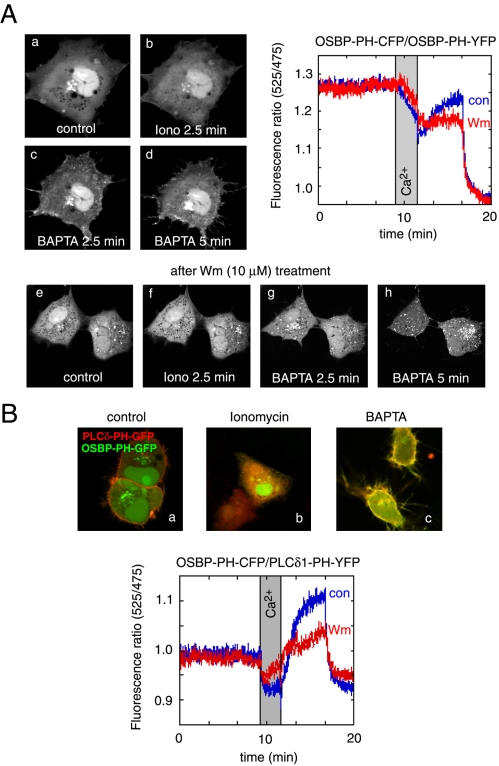
Figure 8.
Effects of cytoplasmic [Ca2+] increases on the distribution of the OSBP-PH-GFP fusion protein. Cells were transfected with the indicated constructs and live cells were examined by confocal microscopy (Zeiss LSM410) at 35°C. (A) Addition of ionomycin (10 μM) in the presence of 2 mM external Ca2+ caused the translocation of the PH domain from the Golgi to the cytoplasm. Chelation of Ca2+ by BAPTA restores the localization in the Golgi and induces a prominent plasma membrane localization of the PH domain. Wortmannin (Wm) pretreatment prevents the plasma membrane localization but not the vesicular localization of the PH domain after ionomycin/BAPTA treatment (A, lower series). FRET measurements between CFP- and YFP-fused OSBP-PH domains coexpressed in COS-7 cells show a high initial FRET value (probably because of the high nuclear accumulation of the constructs), which decreases after ionomycin addition, but shows a rapid increase after Ca2+ chelation, which is mostly sensitive to Wm (10 μM) treatment (A, graph). (B) Simultaneous monitoring of the OSBP-PH and PLCδ1-PH domain translocations by coexpression of GFP and mRFP fused PH domains, respectively. Note the prominent colocalization of the red and green signals after (but not before) the iono/BAPTA treatment. FRET measurements between the same domains fused to CFP and YFP (OSBP-PH-CFP and PLCδ1-PH-YFP) shows a small initial FRET that is abolished after ionomycin treatment and returns well above baseline after Ca2+ chelation. This FRET analysis shows the plasma membrane component of the OSBP-PH movement that is significantly reduced after Wm treatment.