Figure 6.
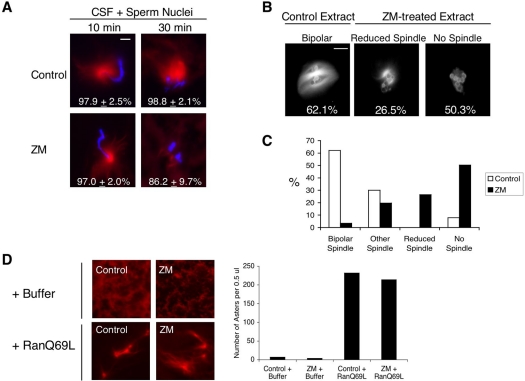
Addition of ZM to egg extracts does not prevent the formation microtubule asters induced from sperm centrosomes or by Ran-GTP, but does reduce chromatin-induced spindle assembly. (A) ZM does not inhibit microtubule nucleation from sperm centrosomes. CSF extracts were supplemented with sperm nuclei (400/μl) and rhodamine-labeled tubulin, and then incubated on ice for 60 min in the presence of either DMSO or 20 μM ZM. Extracts were warmed to 21°C and samples were taken at 10-min intervals and fixed. Microtubules (red) were visualized with rhodamine-tubulin and DNA (blue) with Hoechst 33042 by fluorescence microscopy. Magnification, ×60; bar, 15 μm. The images shown represent the most abundant structures observed in DMSO- and ZM-treated extracts from three independent experiments. (B) ZM reduces the formation of spindles around chromatin-coated beads. Chromatin-coated beads were incubated in CSF extract containing rhodamine-labeled tubulin and either DMSO or 20 μM ZM. Samples were fixed at 60 min, and the status of spindle formation was determined. Magnification, ×60; bar, 15 μm. The images shown represent the most abundant spindle structures observed in DMSO- and ZM-treated extracts. (C) Quantification of spindle structures around chromatin-beads in DMSO- and ZM-treated extracts from B. Samples were fixed 60 min after the initiation of spindle assembly. The type of spindle structures associated with bead clusters that contained at least six beads were assigned to four different categories: “Bipolar Spindles” contained microtubules organized into bipolar structures; “Other” contained microtubules that were not organized into obvious bipolar structures; “Reduced Spindle” contained weak microtubule staining; “No Spindle”, contained no microtubule staining. The graph shown represents one of three independent experiments. More than 150 bead clusters were counted in this experiment. (D) ZM does not inhibit RanQ69L-induced microtubule asters. CSF extracts were supplemented rhodamine-labeled tubulin and then incubated on ice for 60 min in the presence of either DMSO (Control) or 20 μM ZM. Extracts were warmed to 21°C and incubated for an additional 15 min. Buffer or RanQ69L (0.5 mg/ml) was added and samples were taken at 60 min. The total number of microtubule asters was counted.