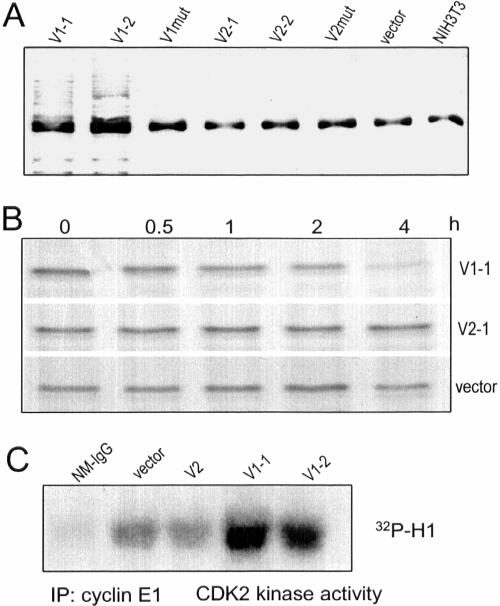
Figure 8.
V1 expression induces p27 ubiquitination and degradation. (A) After immunoprecipitation of p27 from different cell lysate with anti-p27 antibody, the precipitated proteins were analyzed on Western blot probed with antiubiquitin antibody. Ubiquitination of p27 was observed only in V1-transfected cells. The common bands might be the light chain of IgG which was recognized by the secondary antibody used in the Western blot. (B) NIH3T3 fibroblasts, transfected with V1, V2, or the control vector, were incubated with 35S for 1 h. The medium was removed and the cells were washed with DMEM, followed by incubation of the cells in DMEM containing 10% FBS for 0, 0.5, 1, 2, and 4 h. Cell lysate was prepared and immunoprecipitated with anti-p27 antibody. The precipitated proteins were separated by SDS-PAGE, followed by gel-drying and exposure to x-ray film. V1 expression enhanced p27 degradation, but this was not detected in V2- or vector-transfected cells. (C) Cell lysate was immunoprecipitated with anticyclin E antibody E172. The kinase activity of the coprecipitated protein CDK2 was assessed by Western blot incubated with CDK2's substrate histone H1 and 32P. As a control, nonspecific mouse Ig G (NM-IgG) was used for immunoprecipitation. Only the V1-transfected cells produced higher CDK2 activity.