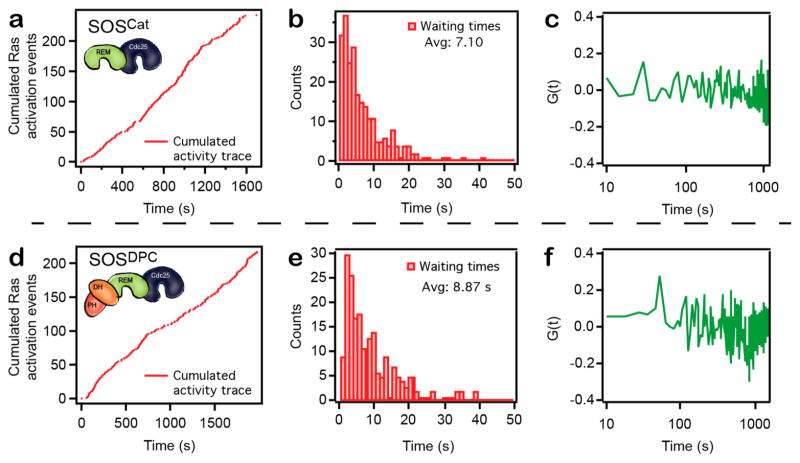
Figure 3.
Single turnover analysis reveals that SOS catalytic states correspond to well-defined conformations of the enzyme. (a) Cumulated Ras activation events as a function of time for an activity trace acquired with the catalytic core of SOS (SOSCat). (b) Waiting time histogram corresponding to data in a. (c) Autocorrelation (G(t) = 〈Δτ(0)Δτ(m)〉 / 〈Δτ2〉, Δτ(m) = τ(m) − 〈τ〉20) of the waiting times corresponding to data shown in a. (d–f) Same data format as in a–c but for an activity trace acquired with SOSDPC, a construct containing the N-terminal DH-PH domains of SOS in addition to the catalytic core. It should be noted that this type of data are intrinsically stochastic and therefore no two traces are the same. Importantly, the overall method is reproducible in its ability to capture these long sequences of Ras activation events.