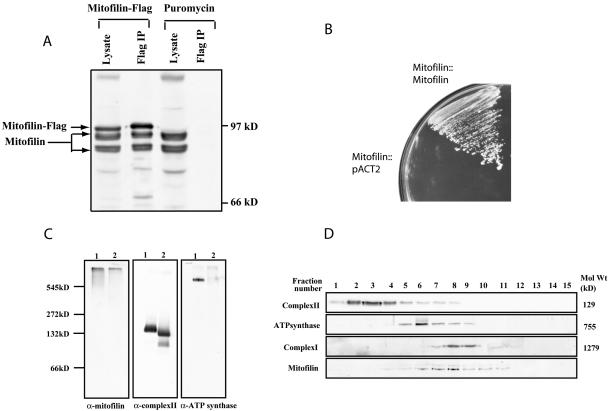
Figure 9.
Mitofilin forms homotypic interactions and assembles into a high-molecular-weight protein complex. (A) Immunoprecipitation of FLAG-tagged mitofilin. Cellular lysate of C2C12 cells expressing mitofilin-FLAG or Puror were immunoprecipitated by an anti-FLAG antibody, and the immunoprecipitates as well as the lysates were subsequently analyzed by Western blotting with an α-mitofilin (cDNA) antibody. (B) The first 67 amino acids of mitofilin were deleted and fused to either the Gal4 DNA binding (pAS2–1) or the activation domains (pACT2) and were cotransformed into the yeast strain AH109 and their growth on triple dropout plates (SD/Leu-Trp-His-) was examined. (C) Characterization of the mitofilin complex by blue native-PAGE. Mouse liver mitochondrial lysates made in 1% digitonin (lane 1) or 1% dodecyl maltoside (lane 2) were separated using 5–16% blue native-PAGE, followed by Western blotting with α-mitofilin (cDNA), anti-complex II, and anti-ATP synthase antibodies. (D) Characterization of the mitofilin complex by glycerol density gradient centrifugation. The 1% digitonin permeabilized mouse liver mitochondrial lysate (1 mg) was separated on a glycerol gradient (10–50%), and fractions collected were analyzed by Western blotting to identify complex I, II, ATP synthase, and the mitofilin complex. Fraction 1 corresponds to the top of the gradient.