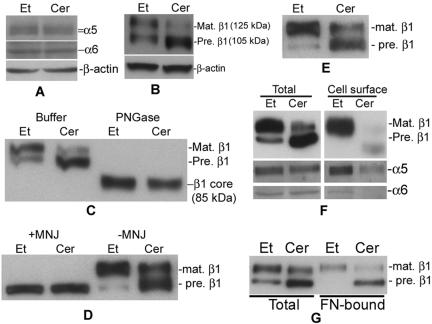
Figure 5.
Treatment with D-e-Cer inhibits glycosylation of β1 integrin in the GC and trafficking of its associated integrin receptors to the cell surface. (A and B) HeLa cells treated with 2.5 μM D-e-Cer (Cer) or Et for 16 h were lysed, and the cell lysates were subjected to Western blot analysis by using antibodies against α5, α6, and β1 integrin subunits. (C) The above-mentioned cell lysates were treated with PGNase F before Western blot analysis. (D) HeLa cells also were treated with D-e-Cer or Et overnight in the presence of MNJ (200 μg/ml) before Western blot analysis. (E) HT1080 cells were treated with 5 μM D-e-Cer or Et and were subjected to Western blot analysis by using anti-β1 integrin antibody. HeLa cells treated with D-e-Cer or Et for 16 h were biotinylated. Total biotinylated cell surface proteins were precipitated by streptavidin-agarose beads and were subjected to Western blot analysis with the antibody against β1, α5, or α6 integrin subunit (F). (G) The lysates from the above-mentioned cells were subjected to in vitro fibronectin binding assays as described under Materials and Methods. The above-mentioned data are representative of the results from at least three experiments. Mat., mature; Pre., precursor. This figure is representative of at least three independent experiments.