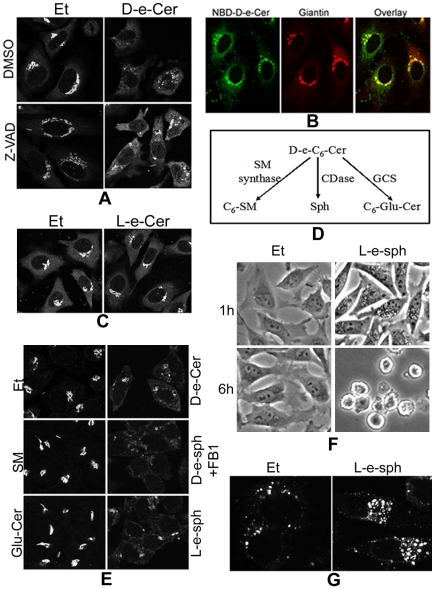
Figure 9.
Sphingosine generated from hydrolysis of ceramide may cause the GC fragmentation via vacuolization. HeLa cells were preincubated with dimethyl sulfoxide (DMSO) or Z-VAD-fmk (50 μM) for 1 h before addition of Et or 2.5 μM D-e-Cer to medium. (B) Sixteen hours later, the cells were fixed and immunostained with the anti-giantin antibody before confocal microscopy (A). Thirty min after incubation with 2.5 μM NBD-C6-cer, HeLa cells were fixed and labeled with the anti-giantin antibody before confocal microscopy. (C) HeLa cells were treated with Et or 10 μM L-e-C6-ceramide (L-e-Cer) for 16 h before immunostaining with giantin and confocal microscopy. (D) Metabolism of ceramide. C6-Glu-Cer, C6-glucosylceramide; CDase, ceramidase; GCS, glucosylceramide synthase; SM, sphingomyelin. (E) HeLa cells were treated with Et, 20 μM D-e-C6-SM, or 20 μM D-e-C8-Glu-Cer for 16 h, and 2.5 μM D-e-sphingosine (D-e-Sph), 2.5 μM L-e-sphingosine (L-e-sph), or 2.5 μM D-e-Cer for 4 h before immunostaining with the anti-giantin antibody and confocal microscopy. (F) HeLa cells treated with Et or 2.5 μM L-e-Sph for 1 h or 6 h were fixed and photographed under a inverted microscope. Note the massive perinuclear vacuolization in the cells treated with L-e-sph for 1 h and total cell rounding within 6 h. The above-mentioned cells treated with Et or L-e-sph for 1 h were stained with the anti-LAMP1 antibody before confocal microscopy (G). Note that the perinuclear vacuoles stained positive with the anti-LAMP1, indicating the lysosomal origin. This figure is representative of at least three independent experiments.