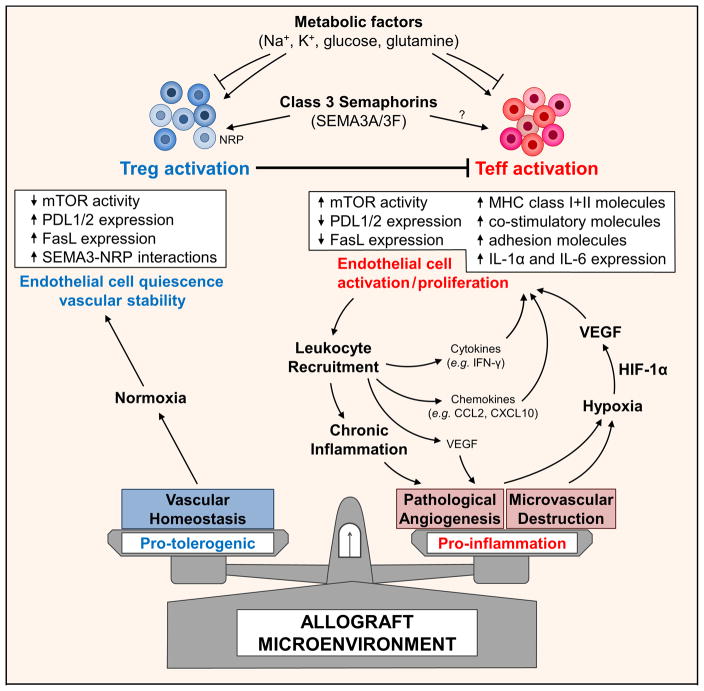
Figure 1. Cartoon illustration of a working model of how the allograft microenvironment may be shaped to support alloimmune tolerance.
Initial inflammatory infiltration into a graft target the microvasculature and may either result in injury or pathological leukocyte-induced angiogenesis. Early injury and microvascular loss results in local tissue ischemia. Uncontrolled pathological angiogenesis also leads to an abnormal microvascular bed, sluggish/chaotic blood flow patterns and may result in tissue hypoxia. Local tissue hypoxia drives HIF-1α-dependent activation of growth factors, cytokines and chemokines (including VEGF-A) but also facilitates the dedifferentiation of T regulatory cells (Teffs) into effectors (Teffs). Within the tumor microenvironment, immune checkpoints expressed on EC, and secreted factors within the microenvironment promote Treg activation, expansion and function, and inhibit T effector cell activity. Also electrolytes and metabolic substrates within the microenvironment regulate local immunity including T regulatory cell responses. Thus, similar to tumors, novel cell surface molecules (e.g. neuropilins (NRP) and secreted molecules, e.g. class 3 semaphorins that are likely to be deficient within allografts have potential to shape the environment and local immunoregulation.