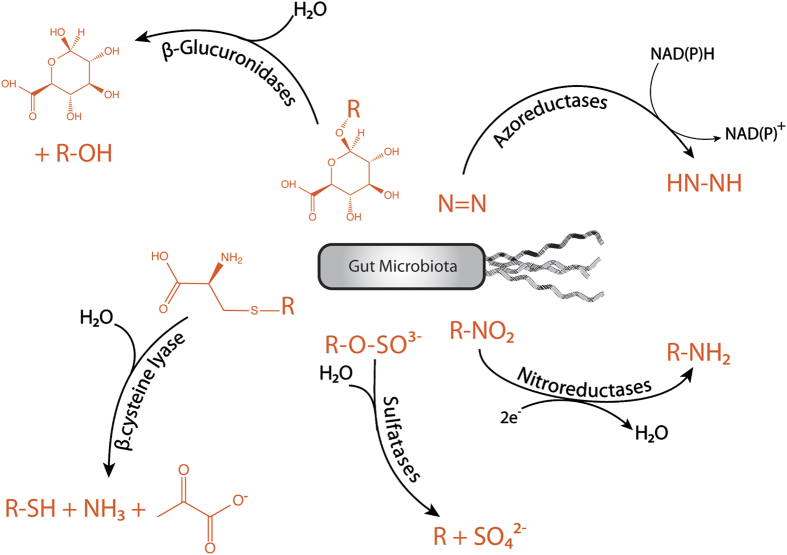
Figure 3.
Xenobiotic-metabolising enzymes of the GI microbiota: (i) The reductive cleavage of azo (N=N) bonds is performed by bacterial azoreductases. Three groups of azoreductases have been described: flavin-dependent NADH preferred azoreductases, flavin-dependent NADPH preferred azoreductases and flavin-free NADPH preferred azoreductases. (ii) Bacterial nitroreductases reduce nitro (–NO2) functional groups to the corresponding amines. Two types of bacterial nitroreductases have been described: type 1 nitroreductases are oxygen-insensitive and catalyse the sequential reduction of nitro groups through the addition of electron pairs from NAD(P)H to produce the nitroso, hydroxylamino and amino derivatives. Type 2 nitroreductases are oxygen-sensitive and catalyse the single-electron reduction of the nitro group to produce a nitro anion radical. (iii) Endogenous sulfate esters are hydrolysed in the GI tract by sulfatases of bacterial origin. (iv) Glutathione conjugates of xenobiotics are also extensively excreted in the bile. They are degradated by various mammalian enzymes (γ-glutamyl transpeptidase and carboxypeptidase), resulting in the formation of cysteine conjugates. These cysteine conjugates may reach part of the GI tract that contain ß-lyase activity and be converted to their corresponding thiol. (v) ß-glucuronidases are present throughout the GI tract and play a role in the hydrolysis of xenobiotic glucuronides, the largest class of xenobiotic conjugates excreted in bile. The intestinal microorganisms are thought to be the major source of ß-glucuronidase because hydrolysis of many xenobiotic glucuronides is dramatically reduced (>90%) in GF or antibiotic-treated rats.