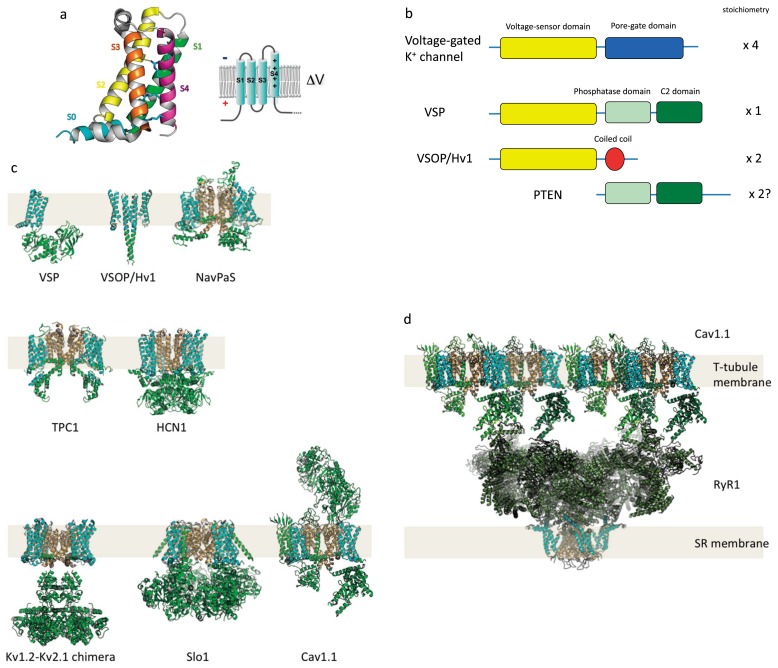
Figure 1.
Structure of VSD and domain organization of VSD-containing proteins.
(a) Structure of Ci-VSD, the voltage-sensor domain of Ci-VSP (Ciona intestinalis voltage-sensing phosphatase) (PDB ID: 4G7V). Right shows a membrane topology of VSD. S4 has multiple positive charges (+) critical for sensing transmembrane voltage. (b) scheme of domain organization of VSP as compared with other related proteins, including voltage-gated potassium channel, voltage-gated proton channel (VSOP/Hv1) and PTEN. (c, d) Atomic structures of VSD-containing proteins are shown as a gallery. VSP: Ci-VSP (full length model based on coordinates from PDB ID:3AWF and PDB ID: 4G7V), VSOP/Hv1: mouse voltage-gated proton channel (dimer model based on a coordinate from PDB ID: 3WKV), NavPaS: insect voltage-gated sodium channel (PDB ID: 5X0M), TPC1: plant two-pore cation channel (PDB ID: 5E1J), HCN1: human Hyperpolarization-activated cyclic nucleotide-gated channel (PDB ID: 5U6O), Kv1.2-Kv2.1 chimera: human voltage-gated potassium channel (PDB ID: 2R9R), Slo1: large conductance calcium activated voltage-gated potassium channel from Aplysia (PDB ID: 5TJ6), Cav1.1: human skeletal muscle-type voltage-gated calcium channel (PDB ID: 5GJV). VSP has a single VSD. VSOP/Hv1 has two VSDs in a dimer. NavAb, Kv1.2-Kv2.1 chimera and Slo1 have four VSDs in a tetramer. TPC1 has four VSDs in a dimer. Cav1.1 has four VSDs in a monomer. (d) Functional unit of excitation-contraction coupling in skeletal muscle cells, which consists of four Cav1.1 subunits and a tetrameric ryanodine receptor calcium-release channel, RyR1 (PDB ID: 3J8H). Auxiliary subunits of Cav1.1 are not shown here. In Cav1.1, motion of the VSD is translated into a structural change in the cytoplasmic domain to induce opening of the RyR pore. This interaction does not require calcium permeation of the Cav1.1 PGD, and direct interaction with the large cytoplasmic domain of RyR plays a central role in this electrochemical coupling. In all pictures, the VSD and PGD are shown as cyan and light orange, respectively.