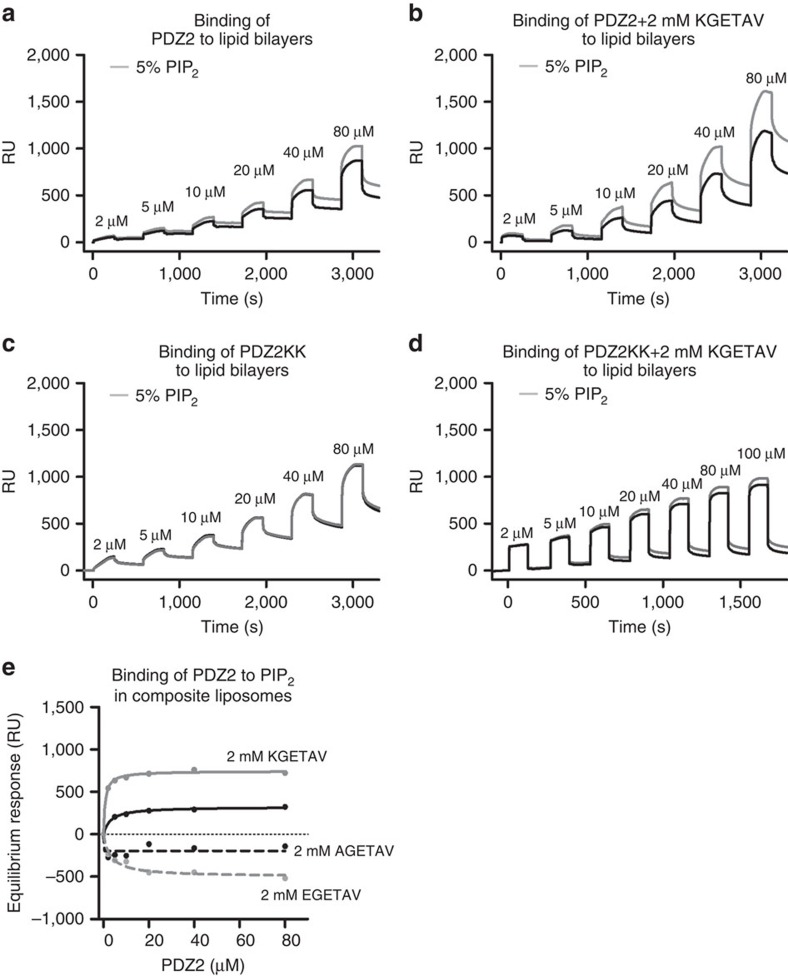
Figure 3. Frizzled 7 peptide enhances the interaction of syntenin PDZ2 with PIP2-containing composite liposomes.
Sensorgrams illustrating the binding of increasing concentrations of PDZ2 wild-type (a,b) or PDZ2 mutant K214A/K250A (c,d) to liposomes containing 30% PC/40% PE/20% PS and either 10% PI (black line) or 5% PI and 5% PIP2 (grey line) in the absence (a,c) or in the presence (b,d) of 2 mM of KGETAV Frizzled 7 peptide. (e) Signals obtained at equilibrium using 5% PIP2-containing liposomes and PDZ2 wild type in the absence (black continuous line) or in the presence of 2 mM of Frizzled 7 peptides (KGETAV, grey continuous line; AGETAV, black dotted line; EGETAV, grey dotted line) were subtracted for association to 10% PI liposomes and plotted as a function of protein concentration. Note that solely the wild-type peptide (KGETAV) enhances PDZ2 interaction with PIP2 embedded in liposomes. The data show one experiment representative of three independent experiments done in duplicate. RU, response units. Supplementary Fig. 3 illustrates that the enhancement of PDZ2 interaction with PIP2 embedded in liposomes in the presence of Frizzled 7 peptide is lost when the PDZ2 carries K214A, K250A or N215D mutations. It also illustrates that PDZ1 in isolation strongly interacts with liposomes, but not with PIP2 embedded in liposomes, even in the presence of Frizzled 7 peptide.