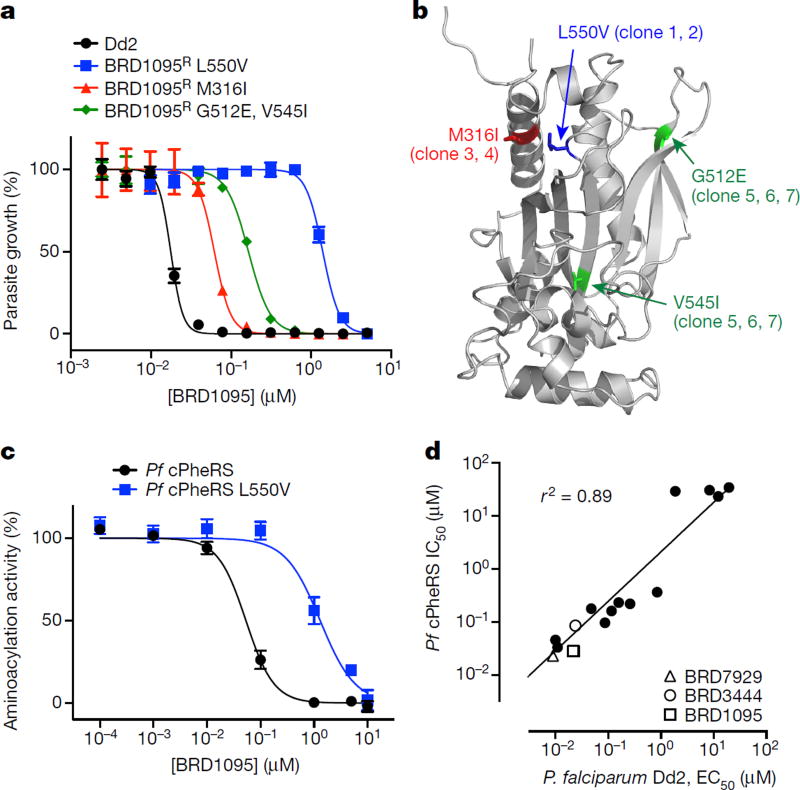
Figure 3. The bicyclic azetidine series targets the cytoplasmic Pf PheRS.
a, P. falciparum Dd2 clones resistant to BRD1095, a derivative of BRD3444 with increased aqueous solubility, were selected in vitro and non-synonymous SNVs were identified via whole-genome sequencing. All clones from three individual flasks contained non-synonymous SNVs within the PF3D7_0109800 locus, which encodes the alpha subunit of the cytoplasmic PheRS. b, The non-synonymous SNVs identified in clones from flask 1 (red), flask 2 (blue), and flask 3 (green) are shown overlaid on a homology model based on the human cytoplasmic PheRS (PDB accession 3L4G) generated in PyMol. c, BRD1095 was assayed against purified recombinant proteins of wild-type cytosolic Pf PheRS and a mutant containing a SNV (giving a L550V substitution), identified from the resistant strain. IC50 value of the wild-type PheRS was 0.045 µM, whereas the IC50 value for BRD1095L550V was 1.30 µM (data are mean ± s.d. for two biological and two technical replicates). d, The bicyclic azetidine series showed a strong correlation between blood-stage growth inhibition and biochemical inhibition of cytosolic Pf PheRS activity. We assayed 15 bicyclic azetidine analogues with varying potency against blood-stage parasites (Dd2 strain) against purified recombinant Pf PheRS. The biochemically derived IC50 values correlate strongly (r2 = 0.89) with the EC50 values determined using the blood-stage growth inhibition assay (see Extended Data Table 2 for structure-activity relationship study and chemical structures).