Abstract
Objective
Cigarette smoking is shown to reduce serum urate. However its impact on risk of gout is unknown. We examined the relationship between cigarette smoking and gout risk prospectively examined in this Asian cohort.
Methods
We analyzed the data from the Singapore Chinese Health Study, a cohort of 63,257 Chinese aged 45–74 years at recruitment in 1993–98. Information on cigarette smoking and other lifestyle factors was collected through in-person interview at recruitment. This analysis included 53,213 participants who participated in either follow-up 1 (1999–2004) and/or follow-up 2 interviews (2006–2010). Cox proportional hazards models were used to assess the relationship between cigarette smoking and gout risk.
Results
A total of 2,244 incident cases of physician-diagnosed gout were identified after a mean follow-up of 11.1 years. Among men, compared to never smokers, the risk of gout in current smokers was decreased by 27% [hazard risk (HR)=0.73; 95% confidence interval (CI)=0.63–0.84]. This risk reduction was greater in lean male smokers (HR=0.69; 95% CI=0.57–0.83) than overweight smokers (HR=0.87; 95% CI=0.67–1.13) (p for interaction=0.09). This inverse association with smoking was rapidly attenuated to become null even in former smokers who had recently quit smoking. Conversely, there was no association between smoking and gout risk in women. In a companion cross-sectional study, current smokers had significantly lower levels of serum urate than former and never smokers, and this observation was present in men and not women.
Conclusion
Current smoking is associated with lower risk of gout in men in this Asian cohort.
Keywords: gout, Cigarette smoking, prospective cohort study, Chinese
Gout, a clinical consequence of hyperuricemia, is the commonest inflammatory arthritides with increasing health burden in Asia and globally.[1–5] Several epidemiologic studies showed that serum urate (SU) levels were lower in cigarette smokers than non-smokers.[6–11] Only one recent publication from the Framingham Heart Study (FHS) cohort has examined the association between smoking and risk of gout and reported a lower risk of gout among smokers, only in men but not in women.[12] However, information on dose and duration of smoking was not available in the FHS. Although this study has adjusted for potential confounders, including obesity, alcohol intake, hypertension and diabetes, dietary risk factors of gout were not adjusted for. This could affect the validity of the findings since smokers are known to have different diet from non-smokers.[13–18] Furthermore, alcohol consumption is an established risk factor for gout, and in populations where alcohol consumption is high, cigarette smoking and alcohol intake are often closely correlated such that residual confounding effect of alcohol cannot be ruled out.
The Singapore Chinese Health Study is a cohort with a wide range of smoking duration and dosage, but rare excessive alcohol intake (usually defined as ≥four drinks per day) against a very low prevalence (11.7%) of weekly or more frequent intake of alcohol (the standard definition of regular alcohol intake).[18] The present study examined the relation of smoking, including smoking intensity and duration, and smoking cessation, with the risk of gout in the middle-aged to elderly Chinese in Singapore.
METHODS
Study population
The Singapore Chinese Health Study is a population-based prospective cohort of 63,257 Chinese adults (27,959 men and 35,298 women) aged 45–74 years at baseline (1993–1998). The study participants, recruited from the public housing estates, were restricted to two major dialect groups in Singapore: Hokkien and Cantonese, who originated from Fujian and Guangdong Provinces in Southern China, respectively. This study was approved by the Institutional Review Boards at the National University of Singapore and the University of Pittsburgh.
Between 1999 and 2004, 52,322 participants were re-contacted for telephone interview at follow-up 1 for an update of lifestyle factors such as height, weight, tobacco use and medical history.[19] At follow-up 2, telephone interview was conducted between 2006 and 2010, 39,528 participants were re-contacted for update of lifestyle factors and medical history (Figure 1). All interviews were tape-recorded and subjected to quality checks by the study investigators.
Figure 1.
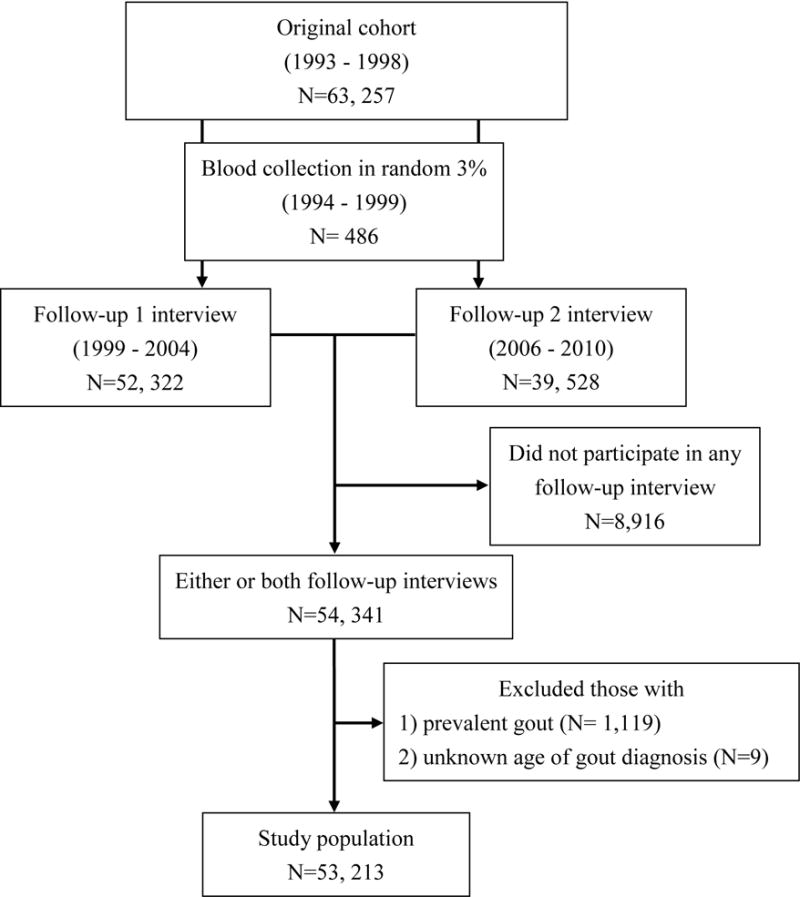
Flowchart of cohort participation
Baseline exposure assessment
At recruitment, each participant was interviewed in person by a trained interviewer using a structured questionnaire, which focused on history of tobacco and alcohol use, dietary habits, physical activity, medical history. Current diet was assessed using a validated 165-item, semi-quantitative food frequency questionnaire. Body weight and height at baseline were self-reported during the interview; and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). There were 9,781 cohort subjects with unknown weight, 97 with unknown height, and 192 with both unknown weight and height. For those with missing weight and/or height, BMI was calculated using imputed weight and/or height derived from the linear regression equation: Weight = intercept + gradient × height, where values for the intercept and gradient were derived from gender-specific regression models based on the entire cohort participants with known heights and weights. This method of imputed BMI was reported in detail previously.[20] Physical activity levels were estimated by questions about numbers of hours per week spent on moderate activities such as brisk walking, bowling, bicycling on level ground, tai chi or chi kung.
Smoking and smoking cessation categories
At baseline interview, the participants were asked, “Have you ever smoked at least one cigarette a day for one year or longer?” Never smokers included those who replied “no”, former smokers included those who answered “yes, but I quit smoking”, and current smokers included those who answered “yes, and I currently smoke”. Both former and current smokers were then asked about number of cigarettes smoked per day (six pre-determined categories: ≤6, 7–12, 13–22, 23–32, 33–42, ≥43) and number of years of smoking (four pre-determined categories: <10, 10–19, 20–39, ≥40). Former smokers were further asked about the duration of cessation (seven pre-determined categories: <1, 1–2, 3–4, 5–9, 10–14, 15–19, ≥20 years). At the follow-up 1 interview, participants were asked the following question, “Have you ever smoked more than 100 cigarettes in your lifetime?” Those who answered “no” were classified as never smokers; those who answered “yes” were asked a second question, “Have you smoked at least one cigarette in the past 30 days?” Those who answered “no” were classified as former smokers and those who answered “yes” were classified as current smokers.
Laboratory measurements
During April 1994 to December 1999, a random 3% of the cohort participants donated blood and single-void urine specimens for research. The biologic specimens used in this analysis of association between SU and smoking were collected from the first 486 subjects (216 men and 270 women) who donated blood. Details of the biospecimen collection, processing and storage procedures have been described.[21] SU was measured using the direct enzymic assay in which urate was oxidized by uricase to allantoin and hydrogen peroxide, and the resultant intensity of the red chromogen measured at 545 nm absorbance.[22]
Incident gout cases
Specifically, at both follow-up interviews, the participants were asked “Have you been told by a doctor that you have gout?” If the response was “yes”, participants were also asked, “Please also tell me the age at which you were first diagnosed?” The interviewers confirmed that the participants had gout but not other arthritis by verifying with the participants that the diagnosis of gout was based on joint pain and swelling attributed to reported hyperuricaemia by their physicians.
A total of 54,341 participants participated in either or both follow-up interviews (52,322 in follow-up 1 and 39,528 in follow-up 2). A major reason for not participating in the follow-up interview was mortality. Hence, as expected, compared to those who participated in at least one follow-up interview, those who did not participate (n=8,916) were older at recruitment (61.1 years versus 55.8 years). They were also more likely to be male and ever smokers, and to have lower education level but a higher prevalence of self-reported hypertension and diabetes at baseline. Participants with prevalent gout diagnosed before baseline (n=1,119) or with missing age of gout diagnosis (n=9) were excluded from this study, leaving 53,213 participants for the current analysis (Figure 1).
Statistical analysis
Person-years for each participant were calculated from date of baseline interview to date of reported gout diagnosis or the latest follow-up interview, whichever occurred first. Cox proportional hazards regression was used to calculate hazard ratio (HR) and its 95% confidence interval (CI) for developing gout by smoking status, duration and dose. All models included age, sex, dialect (Hokkien/Cantonese), year of baseline interview (1993–1995, 1996–1998), educational level (none, primary, secondary or higher), moderate physical activity (<0.5, 0.5–3.9, ≥4 hours/week), alcohol consumption (none, monthly, weekly, daily), BMI (<20.0, 20.0–23.9, 24.0–27.9, ≥28.0 kg/m2), and self-reported history of physician-diagnosed hypertension, diabetes, coronary heart disease (CHD) and stroke at baseline. We also adjusted for dietary intakes of red meat, poultry, fish and shellfish, dairy products, soy foods, non-soy legumes, fruits and vegetables (all in quartiles). These covariates were selected because they were potential or established risk factors of gout previously reported in this cohort or in the literature.[23] Interaction with smoking status was explored for gender and BMI categories (<25 kg/m2 and ≥25 kg/m2) separately. Heterogeneity of the smoking/gout associations was tested with an interaction term (product between smoking status categories and factor of interest) in the models.
The distribution of SU concentration was positively skewed, which was normalized to a large extent by logarithmic transformation. All statistical analyses were performed on the log-transformed values, and we reported their geometric means and corresponding 95% confidence intervals (CI). To examine the relationship between SU and smoking status, we used the multiple linear regression model with adjustment for potential confounders. All statistical analyses were conducted using SAS Version 9.2 (SAS Institute, Inc., Cary, North Carolina). All reported p values are two-sided and p <0.05 was considered statistically significant.
RESULTS
After a mean of 11.1 (SD 3.7) years of follow-up, 2,244 out of 53,213 participants (1,190 at follow-up 1 and 1,054 at follow-up 2) reported to have incident gout. Mean age at diagnosis was 61.4 (SD 8.2, range 45–87) years. Mean time interval between date of interview at diagnosis and diagnosis of gout was 6.3 (SD 3.8, range 1–17) years. The age-adjusted incidence rates of gout were 502 per 100,000 person-years in men and 295 per 100,000 person-years in women.
Compared to never smokers, former and current smokers were more likely to be males, older at recruitment and consume more alcohol. Former smokers had highest prevalence of diabetes, hypertension, CHD and stroke at recruitment while current smokers had the lowest BMI (Table 1). Comparing to non-cases, gout cases were more likely to be male and to have higher BMI, attained higher level of education and consumed more alcohol. Gout cases also had higher prevalence of hypertension and CHD at recruitment (Table 1).
Table 1.
Description of selected characteristics by smoking status and gout [number (%) or mean (SD)], The Singapore Chinese Health Study
| Smoking status | Incident gout status | ||||
|---|---|---|---|---|---|
|
| |||||
| Never | Former | Current | Gout cases | Non-cases | |
| Number of subjects (%) | 38180 | 5439 | 9594 | 2244 | 50969 |
| Male (%) | 9827 (25.7) | 4742 (87.2) | 7841 (81.7) | 1223 (54.5%) | 21187 (41.6%) |
| Cantonese (%) | 18900 (49.5) | 2759 (50.7) | 3781 (39.4) | 1105 (49.2) | 24335 (47.7) |
| Age at recruitment, mean(SD) years | 55.1 (7.7) | 58.7 (8.0) | 56.6 (7.6) | 55.1 (7.4) | 55.8 (7.7) |
| Body mass index, mean(SD) kg/m2 | 23.2 (3.2) | 23.3 (3.3) | 22.5 (3.2) | 24.2 (3.3) | 23.1 (3.2) |
| Level of education (%) | |||||
| No formal education | 10966 (28.7) | 823 (15.1) | 2134 (22.3) | 421 (18.8) | 13502 (26.5) |
| Primary | 15776 (41.3) | 2937 (54.0) | 5078 (52.9) | 1065 (47.4) | 22726 (44.6) |
| Secondary or above | 11428 (30.0) | 1679 (30.9) | 2382 (24.8) | 758 (33.8) | 14741 (28.9) |
| Alcohol consumption (%) | |||||
| None/monthly | 35387 (92.7) | 4442 (81.7) | 7278 (75.9) | 1915 (85.3) | 45192 (88.7) |
| Weekly | 2210 (5.8) | 720 (13.2) | 1431 (14.9) | 239 (10.7) | 4122 (8.1) |
| Daily | 583 (1.5) | 277 (5.1) | 885 (9.2) | 90 (4.0) | 1655 (3.2) |
| Weekly moderate activity (%) | |||||
| <0.5 hours/week | 29519 (77.3) | 3945 (72.5) | 7929 (82.7) | 1675 (74.6) | 39718 (77.9) |
| 0.5–3.9 hours/week | 5517 (14.5) | 905 (16.7) | 1049 (10.9) | 342 (15.3) | 7129 (14.0) |
| ≥4 hours/week | 3144 (8.2) | 589 (10.8) | 616 (6.4) | 227 (10.1) | 4122 (8.1) |
| Diabetes mellitus at baseline (%) | 2864 (7.5) | 588 (10.8) | 601 (6.3) | 3891 (7.6) | 162 (7.2) |
| Hypertension at baseline (%) | 8870 (23.2) | 1499 (27.6) | 1413 (14.7) | 828 (36.9) | 10954 (21.5) |
| Coronary heart disease at baseline (%) | 1076 (2.8) | 396 (7.3) | 295 (3.1) | 116 (5.2) | 1651 (3.2) |
| Stroke at baseline (%) | 364 (0.9) | 117 (2.1) | 87 (0.9) | 17 (0.8) | 551 (1.1) |
All differences among never, former and current smokers were statistically significant (p<0.001).
All differences between gout cases and non-cases were statistically significant (p<0.001) except for dialect (p=0.16) and history of diabetes (p=0.47) and stroke (p=0.14)
Table 2 presents the association between cigarette smoking and gout risk for all cohort subjects and separately among men and women. Compared with never smokers, current smokers had a 20% lower risk of gout (HR 0.80; 95% CI 0.70–0.91). This association was only present in men (HR 0.73; 95% CI 0.63–0.84), but not in women (HR 1.13; 95% CI 0.85–1.51). This sex difference in the smoking-gout association was statistically significant (p for interaction = 0.01). The risk of gout in former smokers was similar to never smokers in both genders.
Table 2.
Cigarette smoking in relation to risk of gout, The Singapore Chinese Health Study, 1993–2010
| Total (n=53,213) | Men (n=22,410) | Women (n=30,803) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cases | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | |
| Smoking status | ||||||
| Never smoker | 1568 | 1.00 | 619 | 1.00 | 949 | 1.00 |
| Former smoker | 317 | 1.04 (0.91–1.19) | 296 | 1.03 (0.90–1.19) | 21 | 1.00 (0.64–1.53) |
| Current smoker | 359 | 0.80 (0.70–0.91) | 308 | 0.73 (0.63–0.84) | 51 | 1.13 (0.85–1.51) |
| Years of smoking among current smokers | ||||||
| Never smoker | 1568 | 1.00 | 619 | 1.00 | 949 | 1.00 |
| < 20 years | 12 | 0.49 (0.28–0.87) | 6 | 0.31 (0.14–0.70) | 6 | 1.06 (0.47–2.36) |
| 20–39 years | 235 | 0.87 (0.75–1.01) | 208 | 0.79 (0.67–0.94) | 27 | 1.23 (0.84–1.81) |
| ≥40 years | 112 | 0.75 (0.61–0.92) | 94 | 0.68 (0.54–0.86) | 18 | 1.05 (0.65–1.69) |
| Number of cigarettes per day among current smokers | ||||||
| Never smoker | 1568 | 1.00 | 619 | 1.00 | 949 | 1.00 |
| 1–12 cig/day | 149 | 0.87 (0.73–1.03) | 114 | 0.77 (0.63–0.95) | 35 | 1.17 (0.83–1.65) |
| 13–22 cig/day | 143 | 0.75 (0.63–0.90) | 129 | 0.69 (0.57–0.84) | 14 | 1.13 (0.67–1.93) |
| ≥23 cig/day | 67 | 0.80 (0.62–1.03) | 65 | 0.76 (0.58–0.99) | 2 | 0.78 (0.19–3.14) |
| Number of years of smoking cessation among former smokers | ||||||
| Never smoker | 1568 | 1.00 | 619 | 1.00 | 949 | 1.00 |
| ≥20 years | 90 | 1.02 (0.82–1.27) | 85 | 1.05 (0.83–1.32) | 5 | 0.75 (0.31–1.81) |
| 5–19 years | 152 | 1.04 (0.87–1.25) | 142 | 1.04 (0.86–1.25) | 10 | 1.13 (0.60–2.12) |
| 1–4 years | 55 | 1.01 (0.76–1.33) | 52 | 1.02 (0.77–1.36) | 3 | 0.72 (0.23–2.24) |
| < 1 year | 20 | 0.93 (0.60–1.46) | 17 | 0.86 (0.53–1.40) | 3 | 1.70 (0.54–5.28) |
All models included age (years), sex (whole cohort), dialect (Hokkien/Cantonese), year of baseline interview (1993–1995, 1996–1998), educational level (none, primary, secondary or higher), moderate physical activity (<0.5, 0.5–3.9, ≥4 hours/week), alcohol consumption (none, monthly, weekly, daily), body mass index (<20.0, 20.0–23.9, 24.0–27.9, ≥28.0 kg/m2), self-reported history of hypertension, diabetes, stroke and coronary heart disease, and dietary intakes of red meat, poultry, fish and shellfish, dairy products, soy foods, non-soy legumes, fruits and vegetables (all in quartiles).
We further investigated the relationship between smoking intensity and duration and gout risk (Table 2). Among male current smokers, the risk of gout was equivalent across different categories of smoking duration or intensity (Table 2). Among former smokers, there was no association between duration of smoking cessation and gout risk. There was no association between smoking intensity, duration or cessation and gout risk among women. We further limited our analysis to those without baseline history of diabetes, hypertension, coronary heart disease and stroke (n=38,306), since these are chronic diseases that are related to smoking. The results remained essentially unchanged; among men, compared to never smokers, the association was reduced in current smokers (HR 0.77; 95% CI 0.64–0.91) but null among former smokers (HR 1.02; 95% CI 0.84–1.24). Among women, the associations were null in both current smokers (HR 1.07; 95% CI 0.73–1.58) and former smokers (HR 0.91; 95% CI 0.47–1.77).
Since obesity is a strong risk factor for gout [24] and current smokers had lower body weight,[18] we further investigated if BMI modified the association between smoking and gout risk among men (Table 3). We used BMI ≥25 kg/m2 as the cut-off to divide the subjects into those with normal weight or overweight/obesity according to the current WHO recommendation.[25] In this analysis, we only included 19,387 men with self-reported body weight and height at baseline. We found a marginally significant interaction between BMI and smoking with risk of gout (p for interaction = 0.09): the associations between current smoking and gout risk were stronger in individuals with BMI <25 kg/m2 (HR 0.69; 95% CI 0.57–0.83) compared to those with BMI ≥25 kg/m2 (HR 0.87; 95% CI 0.67–1.13).
Table 3.
Cigarette smoking in relation to risk of gout among men stratified by body mass index (BMI) level, The Singapore Chinese Health Study, 1993–2010
| BMI <25 kg/m2 (n=14,534) |
BMI ≥25 kg/m2 (n=4,853) |
|||
|---|---|---|---|---|
|
| ||||
| Cases | HRa (95% CI) | Cases | HRa (95% CI) | |
| Smoking status | ||||
| Never smoker | 370 | 1.00 | 193 | 1.00 |
| Former smoker | 167 | 0.99 (0.82–1.19) | 102 | 1.13 (0.89–1.45) |
| Current smoker | 190 | 0.69 (0.57–0.83) | 91 | 0.87 (0.67–1.13) |
| Years of smoking among current smokers | ||||
| Never smoker | 370 | 1.00 | 193 | 1.00 |
| < 19 years | 3 | 0.28 (0.09–0.88) | 2 | 0.30 (0.07–1.21) |
| 20–39 years | 121 | 0.71 (0.57–0.88) | 72 | 1.03 (0.77–1.36) |
| ≥40 years | 66 | 0.72 (0.54–0.96) | 17 | 0.60 (0.36–1.01) |
| Number of cigarettes per day among current smokers | ||||
| Never smoker | 370 | 1.00 | 193 | 1.00 |
| 1–12 cig/day | 76 | 0.77 (0.60–0.99) | 30 | 0.83 (0.56–1.22) |
| 13–22 cig/day | 76 | 0.63 (0.49–0.81) | 43 | 0.92 (0.65–1.30) |
| ≥23 cig/day | 38 | 0.70 (0.49–0.99) | 18 | 0.82 (0.50–1.36) |
All models included age (years), sex (whole cohort), dialect (Hokkien/Cantonese), year of baseline interview (1993–1995, 1996–1998), educational level (none, primary, secondary or higher), moderate physical activity (<0.5, 0.5–3.9, ≥4 hours/week), alcohol consumption (none, monthly, weekly, daily), self-reported history of hypertension, diabetes, stroke and coronary heart disease, and dietary intakes of red meat, poultry, fish and shellfish, dairy products, soy foods, non-soy legumes, fruits and vegetables (all in quartiles).
Since participants may change smoking status over the course of observation period from baseline to follow-up 1, we performed sensitivity analyses with an update of smoking status and other potential confounders fitted as time-dependent covariates in Cox regression analysis, in order to control for change in smoking status, as well as modification in BMI, alcohol consumption and history of diabetes, hypertension, coronary heart disease and stroke during follow-up 1. The results remained essentially the same as the results from using smoking status and other information at baseline interview. Among men, compared to never smokers, only continuous smokers had a decreased HR of 0.72 (95% CI 0.62–0.84), while the association was null among former smokers with HR of 1.08 (0.94–1.24). The reduced risk in current smokers was also stronger among lean men with BMI <25 kg/m2 (HR 0.64; 95% CI 0.54–0.76) compared to those with BMI ≥25 kg/m2 (HR 0.90; 95% CI 0.69–1.17) (p for interaction=0.009). There was no clear association between changes in smoking status and risk of gout among women as a whole or by BMI categories; the HR (95% CI) for former smokers in women was 1.11 (0.77–1.59) and that for current smokers was 1.13 (0.85–1.52).
In the analysis of smoking status and SU, after adjusting for other potential confounders including lifestyle and diet, among men, SU levels were lower in current smokers and former compared to never smokers (p for trend=0.03) (Table 4). The difference in SU levels between current and never smokers in men was statistically significant (p=0.04). SU was higher in overweight men (BMI ≥25 kg/m2) compared to leaner men (BMI <25 kg/m2). However, the association between smoking status and SU was observed in lean men but not in overweight men. Among lean men, current smokers had lower SU levels than never-smokers. We did not observe any material difference in SU levels by dose (comparing 1–12 versus >12 cigarettes per day) or duration of smoking (comparing <20 years to 20 years or more) among men (results not shown). Women had a statistically significant ~25% lower SU concentration than men. There was no material difference in SU levels by smoking status among women (Table 4). Exclusion of four participants who had history of gout with age of diagnosis prior to the blood collection and therefore might be receiving urate-lowering therapy did not alter the results.
Table 4.
The geometric mean of serum urate in μmol/L (95% confidence interval) by smoking status consumption in the Singapore Chinese Health Study
| N | Serum urate (95% CI) | ||
|---|---|---|---|
| Model 1* | Model 2** | ||
| All | |||
|
| |||
| Never smokers | 351 | 339.4 (325.3–354.1) | 340.5 (326.4–355.3) |
| Former smokers | 57 | 326.2 (303.9–350.1) | 328.3 (305.7–352.4) |
| Current smokers | 78 | 323.6 (304.0–344.4) | 323.8 (303.8–345.1) |
| P-value (Trend) | 0.12 | 0.10 | |
|
| |||
| Men | |||
|
| |||
| Never smokers | 95 | 372.7 (352.4–394.2) | 373.7 (353.1–395.4) |
| Former smokers | 55 | 353.5 (330.3–378.3) | 353.7 (329.9–379.1) |
| Current smokers | 66 | 346.3 (325.9–368.0) | 347.2 (325.9–369.8) |
| P-value (Trend) | 0.03 | 0.04 | |
|
| |||
| Lean men (BMI<25 kg/m2) | |||
|
| |||
| Never smokers | 75 | 371.1 (347.9–395.9) | 373.6 (349.7–399.0) |
| Former smokers | 41 | 357.7 (329.7–388.0) | 354.8 (326.3–385.8) |
| Current smokers | 58 | 339.2 (317.4–362.6) | 342.8 (319.9–367.4) |
| P-value (Trend) | 0.02 | 0.03 | |
|
| |||
| Overweight men (BMI ≥25 kg/m2) | |||
|
| |||
| Never smokers | 20 | 393.7 (337.0–459.8) | 387.8 (328.1–458.3) |
| Former smokers | 14 | 353.0 (293.4–424.7) | 375.5 (304.9–462.3) |
| Current smokers | 8 | 380.9 (301.5–481.2) | 387.5 (299.7–501.0) |
| P-value (Trend) | 0.46 | 0.90 | |
|
| |||
| Women | |||
|
| |||
| Never smokers | 256 | 297.7 (273.4–324.2) | 300.8 (275.5–328.3) |
| Former smokers | 2 | 325.9 (236.8–448.4) | 329.8 (238.6–456.0) |
| Current smokers | 12 | 307.8 (264.3–358.4) | 306.8 (262.3–358.8) |
| P-value (Trend) | 0.55 | 0.70 | |
Model adjusted for age at blood taking (years), dialect (Hokkien/Cantonese), educational level (none, primary, secondary or higher), alcohol consumption (none, monthly, weekly, daily), self-reported history of hypertension, diabetes, coronary heart disease and stroke, body mass index (kg/m2) (except in the analysis of lean and overweight men)
Model 2**: further adjusted for dietary intakes of red meat, poultry, fish and shellfish, dairy products, soy foods, non-soy legumes, fruits and vegetables (all in quartiles).
DISCUSSION
The present study represents to date the most comprehensive examination of the relation of smoking, including dose, duration and cessation, using prospective data from a population-based cohort in Asia. The results showed that current smoking was inversely associated with risk of gout in men but not in women. This inverse association with smoking was rapidly attenuated to null even in former smokers who had recently quit smoking for less than a year. SU levels were reduced in current smokers compared to former and never smokers. However, again, this association was only observed in men but not in women. The relations of current smoking with risk of gout and SU were both stronger in lean men compared to their overweight counterparts.
Our findings concur with the observation from the FHS, which showed an inverse association between current smoking and risk of gout in men but a null association in women.[12] Furthermore, this risk reduction among male smokers in our study was not explained by differences in the prevalence of dietary and lifestyle risk factors or comorbidities. The FHS did not examine the effect of dose, duration and cessation on risk of gout. In our study, among male current smokers, the risk of gout was comparable across different categories of smoking duration or intensity.
Several studies have demonstrated that smoking lowers SU.[6–11] Although the FHS also reported lower SU in smokers compared to non-smokers, they did not differentiate the results by gender. In the US Atherosclerosis Risk in Communities study, where 63% of the 8,342 participants were women, the investigators reported a non-statistically significant increase in risk of incident hyperuricemia in current smokers compared to never smokers, without stratification by gender.[26] A cross-sectional study showed that the difference in SU levels between smokers and non-smokers was much greater in men than in women.[6] The Coronary Artery Risk Development in Young Adults study showed that while there were fewer smokers among hyperuricemic men than among normouricemic men, the reverse was observed in women.[27] Hence, our finding of a null effect of smoking on the risk of gout or SU levels in women concurred with these studies. We also showed that the null association in women could not be explained by fewer pack-years among women compared to men, as previously suggested.[12] The differential gender effect of smoking with risk of gout or SU levels deserves further investigation.
Our novel finding is the stronger inverse association between smoking and gout risk/SU in lean compared to overweight men, although SU levels were higher in overweight men overall. Adiposity is one of the strongest risk factor for gout, and adiposity is associated with hyperinsulinemia, which can in turn lead to hyperuricemia through reduced urinary urate clearance.[28 29] Hence, it is plausible that smoking may be less effective in suppressing gout attacks or in lowering SU in overweight men.
Cigarette smoking poses an oxidative stress, and may thereby deplete uric acid, one of the most important anti-oxidant scavengers of reactive oxygen species.[8] Cyanide in tobacco smoking may also inhibit xanthine oxidase responsible for urate formation in purine metabolism.[30 31] Asymptomatic hyperuricemia is a preamble of gout which is characterized by monosodium urate (MSU) crystals deposition. Ultrasound studies have shown presence of MSU deposits in asymptomatic hyperuricemic patients who never experienced gout arthritis.[32 33] MSU crystals have been identified in previously uninvolved joints during the inter-critical stage of gout.[34] Emerging data allude to the possibility of agonistic effect of nicotine on macrophage immune function via alpha 7-nicotinic acetylcholine receptor (α7 nAChR), a major exogenous ligand for nicotine.[35 36] One experimental study using lipopolysaccharide-primed peritoneal mouse and human macrophages showed that α7 nAChRs-signaling inhibits NLRP3 inflammasome activation by preventing mitochondrial DNA release.[37] Hence, in addition to urate-lowering, we propose that the risk reduction by cigarette smoking may also be mediated by the suppression of the intense inflammatory response to MSU. The rapid loss of the inverse association between smoking and gout risk with smoking cessation suggests the reversibility of underpinning mechanisms.
Our study has several strengths. We were able to assess smoking status at recruitment and two separate time-points and therefore ascertain its association with risk of gout more reliably. Other strengths include its large sample size, the prospective study design in eliminating recall bias inherent in case-control studies, and adjustment for relevant lifestyle and diet factors as potential confounders. Conceivable limitations are that the outcome of gout was self-reported and we did not have data on gout treatment and SU levels of all participants. We employed the same case definition used in other large cohort studies whereby gout was defined by “yes” to the question “Have you been told by a doctor to have gout?”.[38 39] Furthermore, our interviewers were trained to enquire if the joint pain and swelling from gout was attributed to reported hyperuricemia by their physicians in order to increase the accuracy of self-reported physician-diagnosed gout. Mandating MSU identification as the gold standard for diagnosis of gout is not practical in population based studies. Utilizing the preliminary American College of Rheumatology criteria may undermine the sensitivity of case definition in primary care setting.[40 41] Using this case definition, we have previously published the association between gout and mortality risk [3], as well as the association between diet and risk of gout [23] from this cohort. Two population-based cohorts in US have also shown that self-report of physician-diagnosed gout could have moderate to good reliability and sensitivity, and could therefore be an adequate proxy for the actual prevalence or incidence in epidemiological studies.[38 42] Finally, we admit that the small number of female smokers may have resulted in the null association between smoking and risk of gout among women.
In conclusion, this study provides epidemiologic evidence for an inverse association between current cigarette smoking and gout risk. While we certainly do not advocate smoking as a means of reducing the risk of gout, further studies are needed to verify this finding and elucidate the suppressive effects of nicotine on the pathophysiology of gout.
SIGNIFICANCE.
Current smoking was associated with reduced risk of gout in men but not in women, and this reduction in risk was attenuated within one year of smoking cessation.
This inverse association with smoking appeared to be stronger in lean smokers than in their more overweight counterparts.
Acknowledgments
We thank Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study and Renwei Wang for the maintenance of the cohort study database. Finally we acknowledge the founding Principal Investigator of the Singapore Chinese Health Study – Mimi C Yu.
FUNDING SOURCES: This study was supported by the National Institutes of Health, USA (NIH R01 CA144034 and UM1 CA182876).
Abbreviations
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- SCHS
Singapore Chinese Health Study
- SD
standard deviation
Footnotes
CONFLICT OF INTEREST STATEMENT
All authors state that they have no conflict of interest.
AUTHORS’ ROLES: Study design: GG Teng, A Pan and WP Koh. Study conduct and data collection: WP Koh and JM Yuan. Data analysis: GG Teng, A Pan and WP Koh. Data interpretation: all authors. Drafting manuscript: GG Teng and WP Koh. Revising manuscript critically for important intellectual content and approving final version of manuscript: all authors. WP Koh takes responsibility for the integrity of the data analysis.
References
- 1.Chang HY, Pan WH, Yeh WT, Tsai KS. Hyperuricemia and gout in Taiwan: results from the Nutritional and Health Survey in Taiwan (1993–96) The Journal of rheumatology. 2001;28(7):1640–6. [PubMed] [Google Scholar]
- 2.Chuang SY, Lee SC, Hsieh YT, Pan WH. Trends in hyperuricemia and gout prevalence: Nutrition and Health Survey in Taiwan from 1993–1996 to 2005–2008. Asia Pacific journal of clinical nutrition. 2011;20(2):301–8. [PubMed] [Google Scholar]
- 3.Teng GG, Ang LW, Saag KG, Yu MC, Yuan JM, Koh WP. Mortality due to coronary heart disease and kidney disease among middle-aged and elderly men and women with gout in the Singapore Chinese Health Study. Annals of the rheumatic diseases. 2011;71(6):924–8. doi: 10.1136/ard.2011.200523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng QY, Chen R, Darmawan J, et al. Rheumatic diseases in China. Arthritis research & therapy. 2008;10(1):R17. doi: 10.1186/ar2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith E, Hoy D, Cross M, et al. The global burden of gout: estimates from the Global Burden of Disease 2010 study. Annals of the rheumatic diseases. 2014;73(8):1470–6. doi: 10.1136/annrheumdis-2013-204647. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 6.Haj Mouhamed D, Ezzaher A, Neffati F, Douki W, Gaha L, Najjar MF. Effect of cigarette smoking on plasma uric acid concentrations. Environmental health and preventive medicine. 2011;16(5):307–12. doi: 10.1007/s12199-010-0198-2. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna BE, Hamed JM, Touhala LM. Serum uric Acid in smokers. Oman medical journal. 2008;23(4):269–74. [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation. 2002;105(10):1155–7. doi: 10.1161/hc1002.105935. [DOI] [PubMed] [Google Scholar]
- 9.Cappuccio FP, Strazzullo P, Farinaro E, Trevisan M. Uric acid metabolism and tubular sodium handling. Results from a population-based study. JAMA. 1993;270(3):354–9. [PubMed] [Google Scholar]
- 10.Lellouch J, Schwartz D, Tran MH. The relationships between smoking and levels of serum urea and uric acid. Results of an epidemiological survey. Journal of chronic diseases. 1969;22(1):9–15. doi: 10.1016/0021-9681(69)90082-4. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Chen S, Shao X, et al. Association of uric acid with metabolic syndrome in men, premenopausal women and postmenopausal women. International journal of environmental research and public health. 2014;11(3):2899–910. doi: 10.3390/ijerph110302899. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Krishnan E. Cigarette smoking is associated with a reduction in the risk of incident gout: results from the Framingham Heart Study original cohort. Rheumatology; Oxford England: 2014. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 13.Bolton-Smith C, Woodward M, Brown CA, Tunstall-Pedoe H. Nutrient intake by duration of ex-smoking in the Scottish Heart Health Study. The British journal of nutrition. 1993;69(2):315–32. doi: 10.1079/bjn19930036. [DOI] [PubMed] [Google Scholar]
- 14.Hebert JR, Kabat GC. Differences in dietary intake associated with smoking status. European journal of clinical nutrition. 1990;44(3):185–93. [PubMed] [Google Scholar]
- 15.McPhillips JB, Eaton CB, Gans KM, et al. Dietary differences in smokers and nonsmokers from two southeastern New England communities. Journal of the American Dietetic Association. 1994;94(3):287–92. doi: 10.1016/0002-8223(94)90370-0. [DOI] [PubMed] [Google Scholar]
- 16.Morabia A, Wynder EL. Dietary habits of smokers, people who never smoked, and exsmokers. The American journal of clinical nutrition. 1990;52(5):933–7. doi: 10.1093/ajcn/52.5.933. [DOI] [PubMed] [Google Scholar]
- 17.Nuttens MC, Romon M, Ruidavets JB, et al. Relationship between smoking and diet: the MONICA-France project. J Intern Med. 1992;231(4):349–56. doi: 10.1111/j.1365-2796.1992.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 18.Koh WP, Yuan JM, Sun CL, Lee HP, Yu MC. Middle-aged and older chinese men and women in singapore who smoke have less healthy diets and lifestyles than nonsmokers. The Journal of nutrition. 2005;135(10):2473–7. doi: 10.1093/jn/135.10.2473. [DOI] [PubMed] [Google Scholar]
- 19.Butler LM, Koh WP, Lee HP, et al. Prospective study of dietary patterns and persistent cough with phlegm among Chinese Singaporeans. American journal of respiratory and critical care medicine. 2006;173(3):264–70. doi: 10.1164/rccm.200506-901OC. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh WP, Yuan JM, Wang R, Lee HP, Yu MC. Body mass index and smoking-related lung cancer risk in the Singapore Chinese Health Study. British journal of cancer. 2010;102(3):610–4. doi: 10.1038/sj.bjc.6605496. doi: 6605496 [pii] 10.1038/sj.bjc.6605496 [doi][published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh WP, Yuan JM, Sun CL, et al. Angiotensin I-converting enzyme (ACE) gene polymorphism and breast cancer risk among Chinese women in Singapore. Cancer research. 2003;63(3):573–8. [PubMed] [Google Scholar]
- 22.Fossati P, Prencipe L, Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of urate in serum and urine. Clinical Chemistry. 1980;26:227–231. [PubMed] [Google Scholar]
- 23.Teng GG, Pan A, Yuan JM, Koh WP. Food Sources of Protein and Risk of Incident Gout in the Singapore Chinese Health Study. Arthritis & rheumatology. 2015;67(7):1933–42. doi: 10.1002/art.39115. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Archives of internal medicine. 2005;165(7):742–8. doi: 10.1001/archinte.165.7.742. [DOI] [PubMed] [Google Scholar]
- 25.WH Organization. Preventing and Managing the Global Epidemic Report on a WHO Consultaion on Obesity. Geneva: World Health Organization; 1997. [PubMed] [Google Scholar]
- 26.McAdams-DeMarco MA, Law A, Maynard JW, Coresh J, Baer AN. Risk factors for incident hyperuricemia during mid-adulthood in African American and white men and women enrolled in the ARIC cohort study. BMC musculoskeletal disorders. 2013;14:347. doi: 10.1186/1471-2474-14-347. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Annals of epidemiology. 1998;8(4):250–61. doi: 10.1016/s1047-2797(97)00204-4. [DOI] [PubMed] [Google Scholar]
- 28.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–11. [PubMed] [Google Scholar]
- 29.Quinones Galvan A, Natali A, Baldi S, et al. Effect of insulin on uric acid excretion in humans. The American journal of physiology. 1995;268(1 Pt 1):E1–5. doi: 10.1152/ajpendo.1995.268.1.E1. [DOI] [PubMed] [Google Scholar]
- 30.Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107(3):416–21. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 31.Massey V, Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. The Journal of biological chemistry. 1970;245(24):6595–8. [PubMed] [Google Scholar]
- 32.Chowalloor PV, Keen HI. A systematic review of ultrasonography in gout and asymptomatic hyperuricaemia. Annals of the rheumatic diseases. 2013;72(5):638–45. doi: 10.1136/annrheumdis-2012-202301. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 33.De Miguel E, Puig JG, Castillo C, Peiteado D, Torres RJ, Martin-Mola E. Diagnosis of gout in patients with asymptomatic hyperuricaemia: a pilot ultrasound study. Annals of the rheumatic diseases. 2012;71(1):157–8. doi: 10.1136/ard.2011.154997. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy TD, Higgens CS, Woodrow DF, Scott JT. Crystal deposition in the knee and great toe joints of asymptomatic gout patients. Journal of the Royal Society of Medicine. 1984;77(9):747–50. doi: 10.1177/014107688407700907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol. 2001;167(11):6518–24. doi: 10.4049/jimmunol.167.11.6518. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8. doi: 10.1038/nature01339. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 37.Lu B, Kwan K, Levine YA, et al. Alpha 7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Molecular medicine. 2014 doi: 10.2119/molmed.2013.00117. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelber AC, Klag MJ, Mead LA, et al. Gout and risk for subsequent coronary heart disease. The Meharry-Hopkins Study. Archives of internal medicine. 1997;157(13):1436–40. [PubMed] [Google Scholar]
- 39.Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH. Long-term cardiovascular mortality among middle-aged men with gout. Archives of internal medicine. 2008;168(10):1104–10. doi: 10.1001/archinte.168.10.1104. [DOI] [PubMed] [Google Scholar]
- 40.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis and rheumatism. 1977;20(3):895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 41.Janssens HJ, Janssen M, van de Lisdonk EH, Fransen J, van Riel PL, van Weel C. Limited validity of the American College of Rheumatology criteria for classifying patients with gout in primary care. Annals of the rheumatic diseases. 2010;69(6):1255–6. doi: 10.1136/ard.2009.123687. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 42.McAdams MA, Maynard JW, Baer AN, et al. Reliability and sensitivity of the self-report of physician-diagnosed gout in the campaign against cancer and heart disease and the atherosclerosis risk in the community cohorts. The Journal of rheumatology. 2011;38(1):135–41. doi: 10.3899/jrheum.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]


