Figure 3.
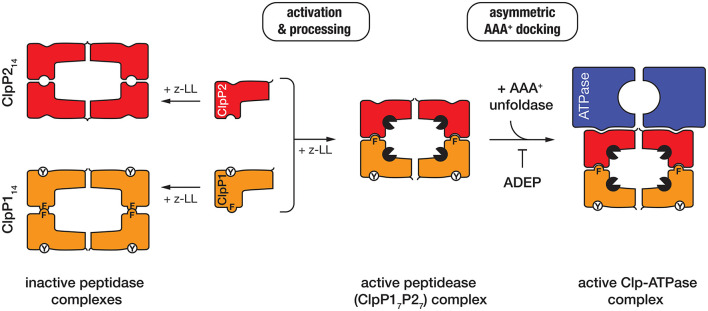
In the presence of the dipeptide activator (z-LL), ClpP1 (orange), and ClpP2 (red) form either homo- (left) or hetero-oligomeric complexes (middle). Activator binding is essential for propeptide processing of both ClpP proteins in Mtb (while only ClpP1 is processed in Msm). Hetero-oligomeric complexes are activated (black packman) through the complementary docking of Phe147 (F) of ClpP1, into a pocket on the handle of ClpP2. In contrast, homo-oligomeric complexes lack this complementary docking and are not active. The unfoldase (blue) docks only to a single face of the active peptidase (i.e., ClpP2) to generate an asymmetric machine. ADEP docks only to the hydrophobic pockets of ClpP2 and as such prevents docking of the unfoldase component.