Fig. 6.
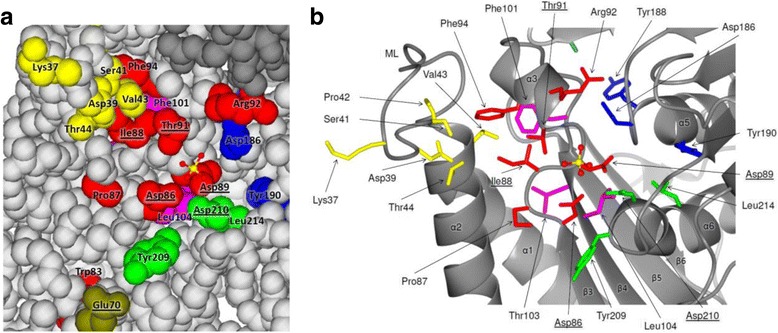
Probable active site of HisNZm depicted as space-filling model (a), illustrating surface exposed residues, and as ribbon diagram with stick representation of selected residues (b) based on the crystal structure of the homodimer as determined by Hwang et al. (2014) [32]. The two monomers are depicted in different gray shades. The side chains of highly conserved residues typical of IMPase-like HolPases are highlighted: yellow = motif 1, olive = motif 2, red = motif 3, pink = motif 4, blue = motif 5, green = motif 6 (compare Fig. 5). The location of the sulfate ion (ball-and-stick model) represents the most likely binding site of the phosphate moiety of the substrate HolP. Key active site residues involved in binding of metal ions (not present in the HisNZm crystal), the substrates phosphate moiety and activation of the water molecule for ester hydrolysis, as derived from the structures of different IMPases [28, 29], are underlined
