Fig. 5.
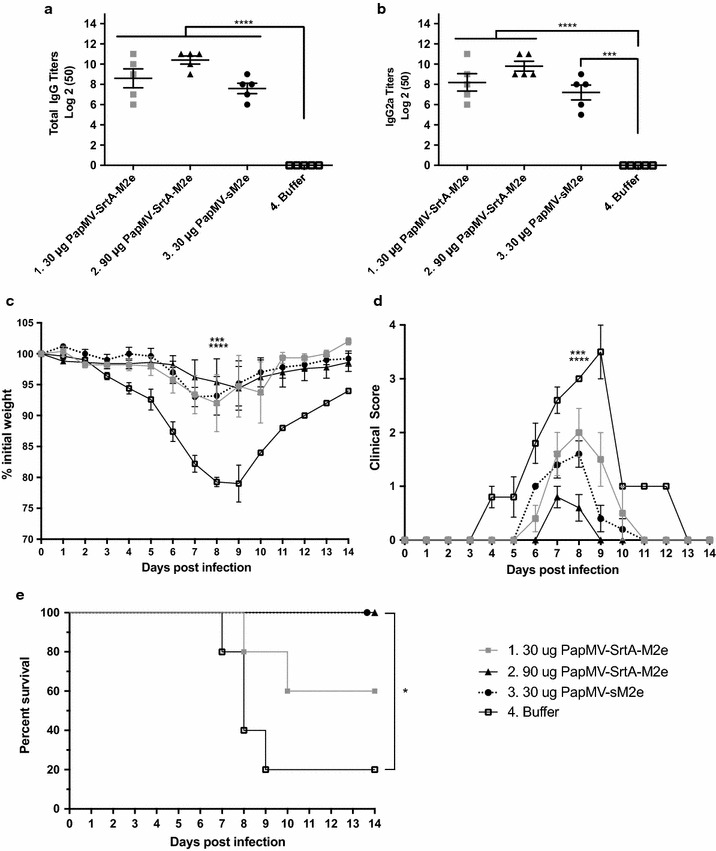
PapMV-SrtA-M2e induces a specific anti-M2e immune response and protection against an influenza challenge. The M2e peptide has been previously shown to be a highly conserved epitope of influenza A virus that is able to induce protection against an influenza challenge. Therefore, Female Balb/C mice (5 per group) were immunized twice with PapMV nanoparticles coupled to the M2e peptide using the sortase (PapMV-SrtA-M2e), PapMV-sM2e [where a small version of the M2e (sM2e) is fused directly to the N-terminus of the PapMV CP] (positive control), or buffer of the vaccine formulation alone. Blood was taken 13 days following the last immunization, and ELISA assays performed to evaluate levels of anti-M2e total IgG (a) or IgG2a (b). **P < 0.01 for groups 2 vs 3 total IgG titers and * P< 0.05 for group 2 vs 3 IgG2a titers. To assess the capacity of the PapMV-SrtA-M2e nanoparticles to induce protection to an influenza challenge, immunized mice were infected with 1 × LD80 of influenza A/WSN/33 virus 14 days after the last immunization, and followed for clinical symptoms and survival for 14 days. c Mean clinical score of infection signs on a scale of 0 to 4. ***P < 0.001 for group 1 vs 4, and for group 2 vs 3, ****P < 0.0001 for groups 2–3 vs 4, and for group 1 vs 2, all at day 8 post-challenge. d Mean weight loss expressed as percentage of initial weight. ***P < 0.001 for group 1 vs 4, and ****P < 0.0001 for group 2–3 vs 4. e Survival of mice expressed as Kaplan–Meier survival curves. *P < 0.5 for groups 2–3 vs 4
