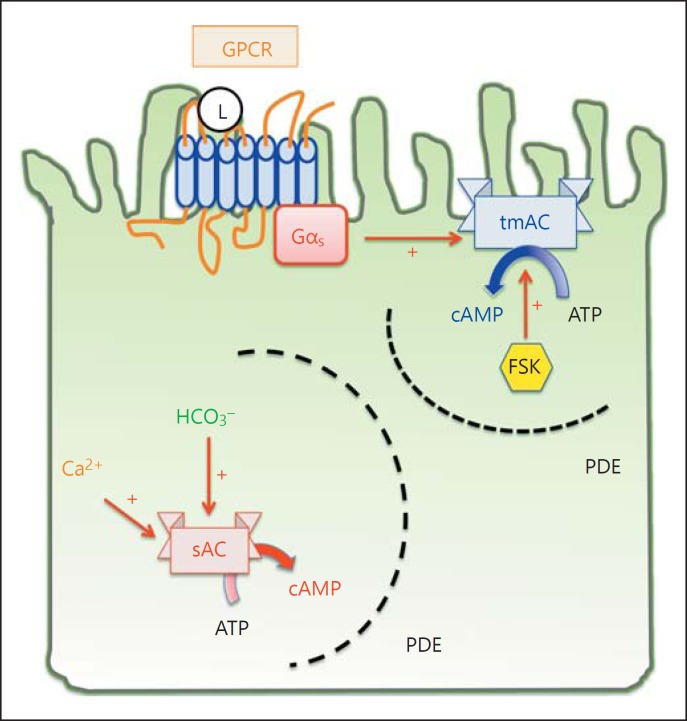
Fig. 1.
Compartmentalization of intracellular cAMP signaling. The classic cAMP signaling takes place at the plasma membrane and begins with a ligand (L) binding to its GPCR on the plasma membrane. The G protein is heterotrimeric, consisting of α, β, γ subunit. Receptor-ligand binding triggers a conformational change and releases the α subunit of the G protein. Depending on the type of α subunit, it can either activate (Gαs, depicted in this figure) or inhibit (Gαi) the activity of tmAC, ADCY1∼9. In contrast to tmAC signaling, sAC takes place in different subcellular compartments, including cytosol, mitochondria and nuclei. sAC is activated by bicarbonate and calcium, but not G protein or the pan-tmAC activator FSK. The specificity of cAMP signaling is made possible by scaffolding proteins that assemble the adenylyl cyclase, cAMP effectors (PKA and Epac), and the substrates in signaling microdomains bordered by cAMP-impermeable membranes or phosphodiesterases, which degrade cAMP.