Abstract
Background:
Dynamic measures, the response to stroke volume (SV) to fluid loading, have been used successfully to guide fluid management decisions in critically ill patients. However, application of dynamic measures is often inaccurate to predict fluid responsiveness in patients with arrhythmias, ventricular dysfunction or spontaneously breathing critically ill patients. Passive leg raising (PLR) is a simple bedside maneuver that may provide an accurate alternative to guide fluid resuscitation in hypovolemic critically ill patients.
Methods:
Pertinent medical literature for fluid responsiveness in the critically ill patient published in English was searched over the past three decades, and then the search was extended as linked citations indicated.
Results:
Thirty-three studies including observational studies, randomized control trials, systemic review, and meta-analysis studies evaluating fluid responsiveness in the critically ill patient met selection criteria.
Conclusions:
PLR coupled with real-time SV monitors is considered a simple, noninvasive, and accurate method to determine fluid responsiveness in critically ill patients with high sensitivity and specificity for a 10% increase in SV. The adverse effect of albumin on the mortality of head trauma patients and chloride-rich crystalloids on mortality and kidney function needs to be considered when choosing the type of fluid for resuscitation.
Keywords: Colloids, critically ill patients, crystalloids, fluid responsiveness, passive leg raising, stroke volume
Introduction
Intravenous fluid administration (IVF) is the cornerstone of the resuscitation therapy in critically ill patients.[1] The appropriateness of the selected intervention to guide the fluid management is often crucial to avoiding the deleterious effect of over, under, or inappropriate resuscitation. Over-resuscitation with fluid may result in pulmonary edema. Under resuscitation would result in hypoperfusion of the tissue with complications of end-organ damage or dysfunction such as renal failure.[2,3]
Traditionally, hemodynamic monitoring parameters such as heart rate, central venous pressure (CVP), and mean arterial pulse pressure (MAP) are often insensitive and sometimes misleading in the assessment of circulating blood volume. Moreover, studies have shown that only approximately 50% of critically ill patients in whom the fluid is administered do exhibit an increase in blood flow and that half of the patients will receive unnecessary fluid loading.[4,5,6,7,8,9,10,11]
Dynamic measures, the response to stroke volume (SV) to fluid loading, have shown to accurately predict fluid responsiveness in critically ill patients as compared to static parameters such as CVP and MAP.[4,12,13] SV variation, when used with controlled mechanical ventilation, is considered a sensitive and specific indicator in determining whether the patient is preload responsive. SV variation is the result of heart-lung interaction.[4,12,13] Depending on the patient's fluid status, the positive pressure breath compressing the inferior and superior vena cava resulting in a decreased venous return, which after transmitting to heart and lungs causes of variation in the SV and the corresponding swing in arterial pressure tracing. Although SV variation is a sensitive indicator of preload responsiveness, it has its limitations. A regular heart rhythm and controlled mechanical ventilation without spontaneous breathing and large tidal volumes >8 mL/kg are required for dynamic parameters to accurately predict volume responsiveness.
Passive leg raising (PLR) in combination with SV is currently considered superior in predicting fluid responsiveness in mechanically ventilated critically ill patients despite cardiac arrhythmias and spontaneous breathing activity compared with dynamic measures such as CVP and MAP.[14,15,16]
This article reviews the pertinent literature describing the use of PLR for fluid responsiveness in critically ill patients with circulatory failure.
Methods
Current medical literature including PubMed, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials and Google Scholar search databases were searched for the primary search terms as fluid resuscitation, fluid responsiveness, and fluids types published in English from April 1989 to October 2016 and then the search was extended as linked citations indicated.
Results
PLR, a simple bedside maneuver, and analysis of the variation in SV-induced by positive pressure ventilation is currently the most accurate and noninvasive method to managing fluid administration in hypovolemic critically ill patients who are mechanically ventilated despite cardiac dysfunctions. There is no convincing evidence to suggest the superiority of colloids over crystalloids fluid resuscitation in hypovolemic critically ill patients.
Discussion
Defining fluid responsiveness
Fluid responsiveness has been defined by at least 10% increase in SV in response to rapid infusion of 20–30 mL/kg or 500 mL of fluids over 10–15 min.[17,18] The resuscitation fluid increases the mean circulating filling pressure greater than the increase in CVP and thereby increasing the gradient for venous return.[14]
Assessment of fluid responsiveness
Static measures of resuscitation fluids
In the static parameters, the fluid responsiveness is arbitrarily defined as an increase in urine output >0.5 mL/kg/h, the CVP of 8–12 mmHg, or the MAP >65 mmHg, following fluid bolus (usually 20–30 mL/kg) over 10–15 min.[19,20] However, these measures are unable to determine fluid responsiveness and may be associated with significant volume overload and higher mortality rate due to aggressive volume expansion.[19,20] Clinical signs such as poor tissue perfusion, low blood pressure, delayed capillary refill, hypotension, tachycardia, and narrow pulse pressure are also unreliable to predict fluid responsiveness.[10] However, several studies have shown a poor correlation between CVP and blood volume changes and no relationship between cardiac filling pressure and fluid volume changes during the resuscitation.[4,5] Further, critically ill patients have limited filling pressure compared with normal individuals when given a similar amount of resuscitation fluids, and nearly half of hemodynamically unstable patients respond to a fluid bolus.[4,5,8,21,22]
Dynamic measures of resuscitation fluids
The gold standard method for assessing volume status and to predict fluid responsiveness is the change in SV following a fluid bolus.[23,24,25,26,27] The use of SV variation to guide fluid resuscitation allows for the appropriate use of fluid and the maximum benefit while mitigating complications. However, there can be considerable variability to different methods of determining SV/cardiac output.
Dynamic parameters can predict changes in SV after a fluid challenge and are based on the interactions between intrathoracic pressure changes with each breath and the left ventricular end-diastolic volume and cardiac output.[17,28,29,30]
As the lung inflate from a positive pressure breath, in the hypovolemic patient the vasculature compresses impeding venous return which is reflected in the variation of the SV which result in a swing in arterial pressure tracing. An SV variation of more than 13% indicates the patient is fluid responsive and should benefit from the additional fluid.[26,27,31] Once the patient has been resuscitated with volume and the SV is <12% and the patient is euvolemic, the positive pressure breath no longer compress the inferior and superior vena cava reducing the venous return and variation of the SV and corresponding arterial pressure tracing is minimize. This will be reflected in an SV reading of <12% indicating another intervention such as inotrope or vasopressor may be selected other than volume.[25,28]
Dynamic parameters can be measured by esophageal Doppler monitoring for aortic blood flow and transthoracic measurements of the left ventricular outflow tract velocities for the estimation of SV. Recently, the use of point-of-care ultrasound (POCS) has been proposed as viable simple and less invasive tool to guide fluid.[28] The validity of the POCS fluid responsiveness guide, however, requires further study to comparing results obtained from various other dynamic methods to further validate the POCS benefits and limitations.
Although dynamic parameters are very sensitive indicator of preload responsiveness, it has several limitations. First, dynamic techniques are invasive, operator dependent, and require considerable expertise,[12,23,25,26,27,31,32,33] second, controlled mechanical ventilation must be present in patients to induce the required changes in SV, third, dynamic parameters were found inaccurate to predict fluid responsiveness in patients with arrhythmias, ventricular dysfunction, and spontaneously breathing critically ill patients with tidal volume <8 mL/kg.[4,12,13]
Passive leg raising
When clinicians are unable to use SV other volume challenge maneuvers can be employed. PLR is a simple bedside technique that can be used to assess fluid responsiveness. This maneuver can be used as a pseudo-fluid challenge of an approximately of 150–300 mL by placing patient head down flat and feet up at a 45° angle. Blood from the lower extremities translocate to intrathoracic compartment where increases right and left ventricular preload and if the patient is responsive increases SV and cardiac output. An increase in SV of more than 10% indicates the patient is preload responsive with the high sensitivity and specificity.
PLR in combination with cardiac output monitoring is the most accurate method to determine fluid responsiveness by tracking changes in SV-induced by positive pressure ventilation in critically ill patients with 86% sensitivity and 90% specificity for a 10% increase in SV.[16] The PRL maneuver is easy to perform taking <5 min to complete and can be repeated after each volume infusion to see if additional volume would be beneficial without any risk of pulmonary edema.[16]
Choices in type of resuscitation fluids
There is no convincing evidence to suggest the superiority of colloids over crystalloids fluid resuscitation in hypovolemic critically ill patients.[33,34,35,36,37,38,39]
A choice between colloids and crystalloids and the different impact of chloride-rich versus balanced solutions can impact the patient outcomes receiving fluid resuscitation. Albumin and 0.9% sodium chloride (NaCl) appear to have similar effects on mortality in critically ill patients, with the exception of patients with traumatic brain injury [Table 1].[40]
Table 1.
Comparison of different colloid and crystalloid fluids with plasma
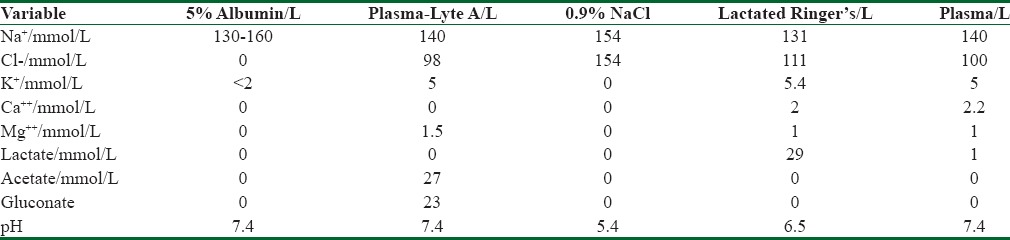
The 2004 Saline versus Albumin Fluid Evaluation trial is the largest clinical trial to date comparing 5% albumin and 0.9% NaCl in a heterogeneous group of 6997 adult patients requiring fluid resuscitation. Authors found no difference in the primary outcome of 28-day all-cause mortality between the albumin and crystalloids, but in the 492 (7%) patients with traumatic brain injuries, the relative risk of death was significantly greater with albumin compared to saline. Further, analyses by the same investigators indicated that this detrimental effect was limited only to patients with severe traumatic brain injuries. While crystalloid appears to be the fluid of choice for trauma patients, colloid is a more protective IVF among patients with severe infection and septic shock.[37,40,41]
Colloid solutions
Human albumin (5%) in saline is the most frequently used colloids in clinical practice. The oncotic pressure gradient generated by albumin infusion will draw fluid from interstitial fluid into the intravascular space, therefore, increasing the efficiency of intravascular expansion relative to a comparable amount of crystalloid.[17] Although albumin is safe for use as a resuscitation fluid in patients with sepsis, its use is associated with increased mortality among patients with traumatic brain injury [Table 1].[40]
The use of hydroxyethyl starch solutions (HES), a synthetic colloid, is associated with high rates of acute kidney injury (AKI) and renal replacement therapy among patients with volume shock.[42,43] Thus, the current guideline discourages the use of HES in patients with volume shock due to higher rates of mortality.[44,45,46,47]
Crystalloid solutions
Balanced salt solutions have shown mortality and morbidity benefits over normal saline in critically ill patients.[28,46,47] Normal saline (0.9% NaCl) is the most widely used crystalloids for the resuscitation in the critically ill patients. The use of normal saline in large amount has been shown to induce renal vasoconstriction, AKI, nonanion gap metabolic acidosis, and increased inflammatory cytokines release.[48,49,50,51]
Optimal dosing of fluid therapy after resuscitation
Maintenance fluid therapy should be used in volume-responsive patients when end-organ perfusion goals are met. Patients with hypovolemic shock are often acute renal injury and excrete hypotonic urine because of a defect in urinary diluting ability. Healthy children and adults should be able to lower urinary osmolality below 100 mOsm/kg. The daily solute load that needs to be excreted in adults on a normal diet is 500–1000 mOsm, which consists of urea generated from metabolism of dietary protein, and electrolytes.[52]
Patients with circulatory failure are unable to lower urine osmolality below 100 mOsm/kg. The low solute intake in such patients who already have a urinary dilution defect will lead to increased free water retention and hyponatremia.[53] Therefore, a strategy to determine appropriate maintenance fluid dosing is needed to avoid volume overload and hyponatremia after the initial fluid resuscitation. In this strategy, the electrolyte-free water clearance is dependent on the obligate urine output that, in turn, is dependent on the daily solute load that needs to be excreted.[53]
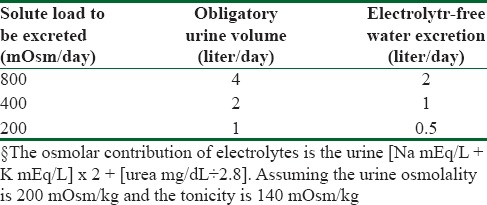
To appreciate this, consider a hypothetical patient who is unable to lower urine osmolality below 200 mOsm/kg and has a urine Na+ concentration of 50 mEq/L (osmolality 100 mOsm/kg), K+ 20 mEq/L (osmolality 40 mOsm/kg), and urea168 mg/dL (osmolality 60 mOsm/kg). The effective osmolality or tonicity of the urine is 140 mOsm/kg (the sum of the Na+, and K+, osmolality) since urea is an ineffective solute. Thus, the urine in this particular patient is half isotonic compared with plasma osmolality, or one can say each liter of urine contains 0.5 L of free water.
If this hypothetical patient had a daily solute load of 800 mOsm, he would excrete 4 L obligate urine daily (800 mOsm/200 mOsm/kg) and hence 2.0 L of electrolyte-free water clearance (approximately half of the serum tonicity). This patient would be highly unlikely to become hyponatremic with a normal daily water intake of 2.5 L. If, on the other hand, the daily solute load were lowered to 400 mOsm, the daily obligatory urine output would be 2.0 L (400 mOsm/200 mOsm/kg), the daily electrolyte-free water excretion would be only 1.0. In this situation, a restriction of water intake to approximately 1.5 L daily would be needed to avoid progressive hyponatremia.[54] Finally, a patient with a daily solute load of 200 mOsm would have a daily urine output of 1 L (200 mOsm/200 mOsm/kg) and electrolyte-free water excretion of 0.5 L and would likely become progressively hyponatremic despite severe restriction of water intake[54,55] [Table 2].
Table 2.
Calculation of obligatory urine volume and electrolyte-free water excretion
Conclusions
PLR, when used with SV variation, is a highly sensitive and specific indicator in predicting fluid responsiveness in critically ill patients who are mechanically ventilated despite cardiac dysfunctions. There is no convincing evidence to suggest the superiority of colloids over crystalloids fluid resuscitation in hypovolemic critically ill patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Guidelines for Intensive Care Unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27:633–8. [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 3.Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015;43:68–73. doi: 10.1097/SHK.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–65. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 5.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: A critical analysis of the evidence. Chest. 2002;121:2000–8. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 6.Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: Data from the prospective FINNAKI study. Crit Care. 2012;16:R197. doi: 10.1186/cc11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: The FENICE study: A global inception cohort study. Intensive Care Med. 2015;41:1529–37. doi: 10.1007/s00134-015-3850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensive Care Med. 2016;42:1935–47. doi: 10.1007/s00134-015-4134-1. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, Pelosi P, Pearse R, Payen D, Perel A, Hoeft A, et al. Perioperative cardiovascular monitoring of high-risk patients: A consensus of 12. Crit Care. 2015;19:224. doi: 10.1186/s13054-015-0932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saugel B, Ringmaier S, Holzapfel K, Schuster T, Phillip V, Schmid RM, et al. Physical examination, central venous pressure, and chest radiography for the prediction of transpulmonary thermodilution-derived hemodynamic parameters in critically ill patients: A prospective trial. J Crit Care. 2011;26:402–10. doi: 10.1016/j.jcrc.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Monge García MI, Guijo González P, Gracia Romero M, Gil Cano A, Oscier C, Rhodes A, et al. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med. 2015;41:1247–55. doi: 10.1007/s00134-015-3898-7. [DOI] [PubMed] [Google Scholar]
- 12.Corl K, Napoli AM, Gardiner F. Bedside sonographic measurement of the inferior vena cava caval index is a poor predictor of fluid responsiveness in emergency department patients. Emerg Med Australas. 2012;24:534–9. doi: 10.1111/j.1742-6723.2012.01596.x. [DOI] [PubMed] [Google Scholar]
- 13.Yong Y, Wu D, Fernandes V, Kopelen HA, Shimoni S, Nagueh SF, et al. Diagnostic accuracy and cost-effectiveness of contrast echocardiography on evaluation of cardiac function in technically very difficult patients in the Intensive Care Unit. Am J Cardiol. 2002;89:711–8. doi: 10.1016/s0002-9149(01)02344-x. [DOI] [PubMed] [Google Scholar]
- 14.De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: Influence of tidal volume. Intensive Care Med. 2005;31:517–23. doi: 10.1007/s00134-005-2586-4. [DOI] [PubMed] [Google Scholar]
- 15.Reuter DA, Bayerlein J, Goepfert MS, Weis FC, Kilger E, Lamm P, et al. Influence of tidal volume on left ventricular stroke volume variation measured by pulse contour analysis in mechanically ventilated patients. Intensive Care Med. 2003;29:476–80. doi: 10.1007/s00134-003-1649-7. [DOI] [PubMed] [Google Scholar]
- 16.Monnet X, Teboul JL. Passive leg raising: Five rules, not a drop of fluid! Crit Care. 2015;14:19. doi: 10.1186/s13054-014-0708-5. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein S, Bagshaw S, Cecconi M, Okusa M, Wang H, Kellum J, et al. Pharmacological management of fluid overload. Br J Anaesth. 2014;113:756–63. doi: 10.1093/bja/aeu299. [DOI] [PubMed] [Google Scholar]
- 18.Cherpanath TG, Geerts BF, Lagrand WK, Schultz MJ, Groeneveld AB. Basic concepts of fluid responsiveness. Neth Heart J. 2013;21:530–6. doi: 10.1007/s12471-013-0487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoste EA, Maitland K, Brudney CS, Mehta R, Vincent JL, Yates D, et al. Four phases of intravenous fluid therapy: A conceptual model. Br J Anaesth. 2014;113:740–7. doi: 10.1093/bja/aeu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuire MD, Heung M. Fluid as a drug: Balancing resuscitation and fluid overload in the intensive care setting. Adv Chronic Kidney Dis. 2016;23:152–9. doi: 10.1053/j.ackd.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–81. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- 22.Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care. 2014;4:21. doi: 10.1186/s13613-014-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marik PE. Techniques for assessment of intravascular volume in critically ill patients. J Intensive Care Med. 2009;24:329–37. doi: 10.1177/0885066609340640. [DOI] [PubMed] [Google Scholar]
- 24.Peake SL, Delaney A, Bellomo R ARISE Investigators. Goal-directed resuscitation in septic shock. N Engl J Med. 2015;372:190–1. doi: 10.1056/NEJMc1413936. [DOI] [PubMed] [Google Scholar]
- 25.Natalini G, Rosano A, Taranto M, Faggian B, Vittorielli E, Bernardini A. Arterial versus plethysmographic dynamic indices to test responsiveness for testing fluid administration in hypotensive patients: A clinical trial. Anesth Analg. 2006;103:1478–84. doi: 10.1213/01.ane.0000246811.88524.75. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–402. doi: 10.1213/ANE.0b013e3181eeaae5. [DOI] [PubMed] [Google Scholar]
- 27.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 28.Lee CW, Kory PD, Arntfield RT. Development of a fluid resuscitation protocol using inferior vena cava and lung ultrasound. J Crit Care. 2016;31:96–100. doi: 10.1016/j.jcrc.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Lira A, Pinsky MR. Choices in fluid type and volume during resuscitation: Impact on patient outcomes. Ann Intensive Care. 2014;4:38. doi: 10.1186/s13613-014-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97:820–6. doi: 10.1097/00000542-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Marik PE. Fluid responsiveness and the six guiding principles of fluid resuscitation. Crit Care Med. 2016;44:1920–2. doi: 10.1097/CCM.0000000000001483. [DOI] [PubMed] [Google Scholar]
- 32.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–95. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 33.Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100:1093–106. doi: 10.1213/01.ANE.0000148691.33690.AC. [DOI] [PubMed] [Google Scholar]
- 34.Reich DL, Konstadt SN, Raissi S, Hubbard M, Thys DM. Trendelenburg position and passive leg raising do not significantly improve cardiopulmonary performance in the anesthetized patient with coronary artery disease. Crit Care Med. 1989;17:313–7. doi: 10.1097/00003246-198904000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Boulain T, Achard JM, Teboul JL, Richard C, Perrotin D, Ginies G. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest. 2002;121:1245–52. doi: 10.1378/chest.121.4.1245. [DOI] [PubMed] [Google Scholar]
- 36.Roeck M, Jakob SM, Boehlen T, Brander L, Knuesel R, Takala J. Change in stroke volume in response to fluid challenge: Assessment using esophageal Doppler. Intensive Care Med. 2003;29:1729–35. doi: 10.1007/s00134-003-1720-4. [DOI] [PubMed] [Google Scholar]
- 37.Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, et al. SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–84. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- 38.Yunos NM, Bellomo R, Story D, Kellum J. Bench-to-bedside review: Chloride in critical illness. Crit Care. 2010;14:226. doi: 10.1186/cc9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finfer S, McEvoy S, Bellomo R, McArthur C, Myburgh J, et al. SAFE Study Investigators. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med. 2011;37:86–96. doi: 10.1007/s00134-010-2039-6. [DOI] [PubMed] [Google Scholar]
- 40.Rochwerg B, Alhazzani W, Sindi A, Heels-Ansdell D, Thabane L, Fox-Robichaud A, et al. Fluid resuscitation in sepsis: A systematic review and network meta-analysis. Ann Intern Med. 2014;161:347–55. doi: 10.7326/M14-0178. [DOI] [PubMed] [Google Scholar]
- 41.Reinhart K, Perner A, Sprung CL, Jaeschke R, Schortgen F, Johan Groeneveld AB, et al. Consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med. 2012;38:368–83. doi: 10.1007/s00134-012-2472-9. [DOI] [PubMed] [Google Scholar]
- 42.Wiedermann CJ, Joannidis M. Accumulation of hydroxyethyl starch in human and animal tissues: A systematic review. Intensive Care Med. 2014;40:160–70. doi: 10.1007/s00134-013-3156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christidis C, Mal F, Ramos J, Senejoux A, Callard P, Navarro R, et al. Worsening of hepatic dysfunction as a consequence of repeated hydroxyethylstarch infusions. J Hepatol. 2001;35:726–32. doi: 10.1016/s0168-8278(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 44.Bayer O, Reinhart K, Sakr Y, Kabisch B, Kohl M, Riedemann NC, et al. Renal effects of synthetic colloids and crystalloids in patients with severe sepsis: A prospective sequential comparison. Crit Care Med. 2011;39:1335–42. doi: 10.1097/CCM.0b013e318212096a. [DOI] [PubMed] [Google Scholar]
- 45.Disma N, Mameli L, Pistorio A, Davidson A, Barabino P, Locatelli BG, et al. A novel balanced isotonic sodium solution vs normal saline during major surgery in children up to 36 months: A multicenter RCT. Paediatr Anaesth. 2014;24:980–6. doi: 10.1111/pan.12439. [DOI] [PubMed] [Google Scholar]
- 46.Allen SJ. Fluid therapy and outcome: Balance is best. J Extra Corpor Technol. 2014;46:28–32. [PMC free article] [PubMed] [Google Scholar]
- 47.Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the Intensive Care Unit: The SPLIT randomized clinical trial. JAMA. 2015;314:1701–10. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 48.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte ® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 49.Kellum JA. Saline-induced hyperchloremic metabolic acidosis. Crit Care Med. 2002;30:259–61. doi: 10.1097/00003246-200201000-00046. [DOI] [PubMed] [Google Scholar]
- 50.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–35. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19:1076–8. doi: 10.1681/ASN.2007091042. [DOI] [PubMed] [Google Scholar]
- 52.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–9. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 53.Sands JM, Layton HE. Advances in understanding the urine-concentrating mechanism. Annu Rev Physiol. 2014;76:387–409. doi: 10.1146/annurev-physiol-021113-170350. [DOI] [PubMed] [Google Scholar]
- 54.Corona G, Giuliani C, Parenti G, Norello D, Verbalis JG, Forti G, et al. Moderate hyponatremia is associated with increased risk of mortality: Evidence from a meta-analysis. PLoS One. 2013;8:e80451. doi: 10.1371/journal.pone.0080451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Assadi F. Hyponatremia: A problem-solving approach to clinical cases. J Nephrol. 2012;25:473–80. doi: 10.5301/jn.5000060. [DOI] [PubMed] [Google Scholar]


