Table 3.
Coupling yields and TMEM16A inhibition of AACT compounds (10aa–10bw). Yields (%) are of the isolated or purified products. IC50 (μM) for inhibition of TMEM16A anion conductance using a fluorescence plate reader (FPR) assay (SEM in parentheses; n = 3). Purity of assayed compounds was >95% based on HPLC-LCMS analysis at 254 nm, and absence of impurities was confirmed by inspection of 1H NMR spectra. aLow solubility in DMSO.
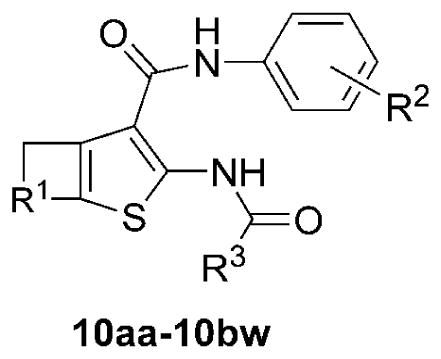
| ||||||
|---|---|---|---|---|---|---|
| Product Final | SM Amino thiophene | R1 | R2 | R3 | Isolated Yield (%) | FPR IC50 TMEM16A (μM) |
| (based on cycloheptanone 7a) | ||||||
| 10aa | 9a | -(CH2)4- | 2-(CH3) | CF3 | 61 | 0.42 (0.03) |
| 10ab | 9a | -(CH2)4- | 2-(CH3) | CF2CF3 | 65 | 1.3 |
| 10ac | 9a | -(CH2)4- | 2-(CH3) | CH3 | 82 | >10 |
| 10ad | 9a | -(CH2)4- | 2-(CH3) | CH2CH3 | 70 | >20 |
| 10ae | 9b | -(CH2)4- | H | CF3 | 38 | 0.3 (0.005) |
| 10af | 9b | -(CH2)4- | H | CF2CF3 | 97 | 2.5 |
| 10ag | 9b | -(CH2)4- | H | CH3 | 80 | >20 |
| 10ah | 9b | -(CH2)4- | H | CH2CH3 | 87 | 1.2 |
| 10ai | 9c | -(CH2)4- | 2-(CH2CH3) | CF3 | 6 | 1.3 (0.7) |
| 10aj | 9d | -(CH2)4- | 2-F | CF3 | 48 | 1.3 |
| 10ak | 9e | -(CH2)4- | 4-F | CF3 | 60 | 0.32 (0.11) |
| 10al | 9f | -(CH2)4- | 2-Cl | CF3 | 87 | 0.66 (0.02) |
| 10am | 9f | -(CH2)4- | 2-Cl | CF2CF3 | 91 | >20 |
| 10an | 9g | -(CH2)4- | 3-Cl | CF3 | 23 | 5 (0.2) |
| 10ao | 9h | -(CH2)4- | 4-Cl | CF3 | 4 | 3 (0.09) |
| 10ap | 9i | -(CH2)4- | 4-(CF3) | CF3 | 2 | 5 (0.10) |
| 10aq | 9j | -(CH2)4- | 2-(OCF3) | CF3 | 12 | 1.3 (0.2) |
| 10ar | 9j | -(CH2)4- | 2-(OCF3) | CF2CF3 | 30 | 2.7 (0.07) |
| 10as | 9j | -(CH2)4- | 2-(OCF3) | CH3 | 55 | >20 |
| 10at | 9j | -(CH2)4- | 2-(OCF3) | CH2CH3 | 50 | >20 |
| (based on cyclohexanone 7b) | ||||||
| 10au | 9k | -(CH2)3- | H | CF3 | 93 | 0.37 (0.01) |
| 10av | 9l | -(CH2)3- | 2-(CH3) | CF3 | 17 | 0.17 (0.001) |
| 10aw | 9m | -(CH2)3- | 4-(CH3) | CF3 | 30 | 0.22 (0.01) |
| 10ax | 9n | -(CH2)3- | 4-F | CF3 | 48 | 0.49 (0.02) |
| (based on tetrahydro-4H-pyran-4-one 7c) | ||||||
| 10ay | 9o | -CH2OCH2- | 2-(CH3) | CF2CF3 | 6 | 1.6 (0.09) |
| 10az | 9o | -CH2OCH2- | 2-(CH3) | CH2CH3 | 40 | 3 (0.09) |
| 10ba | 9p | -CH2OCH2- | 2-Cl | CF3 | 67 | 1.3 |
| 10bb | 9q | -CH2OCH2- | 4-(CH3) | CF3 | 38 | 5 (0.1) |
| 10bc | 9r | -CH2OCH2- | 4-F | CF3 | 15 | 3.8 (0.09) |
| (based on cyclopentanone 7d) | ||||||
| 10bd | 9s | -(CH2)2- | 2-(CH3) | CF3 | 10 | >20 |
| 10be | 9s | -(CH2)2- | 2-(CH3) | CF2CF3 | 37 | 6.2 |
| 10bf | 9s | -(CH2)2- | 2-(CH3) | CH3 | 38 | >20 |
| 10bg | 9s | -(CH2)2- | 2-(CH3) | CH2CH3 | 33 | >20 |
| 10bh | 9t | -(CH2)2- | 4-(CH3) | CF3 | 77 | 2.5 (0.04) |
| 10bi | 9u | -(CH2)2- | 2-Cl | CF3 | 64 | 1.3 |
| 10bj | 9v | -(CH2)2- | 2-(OCF3) | CF3 | 56 | 0.37 (0.02) |
| (2nd-generation inhibitors with novel R3 substituents, including chloro/bromo/iodo difluoroacetyl) | ||||||
| 10bk | 9a | -(CH2)4- | 2-(CH3) | CF2Cl | 27 | 0.18 (0.008) |
| 10bl | 9a | -(CH2)4- | 2-(CH3) | CF2CF2CF3 | 61 | 0.38 (0.01) |
| 10bm | 9a | -(CH2)4- | 2-(CH3) | CF2Br | 36 | 0.083 (0.007) |
| 10bn | 9a | -(CH2)4- | 2-(CH3) | CF2I | 67 | 0.6 (0.02) |
| 10bo | 9b | -(CH2)4- | H | CF2Cl | 16 | 0.925 (0.002) |
| 10bp | 9b | -(CH2)4- | H | CF2Br | 15 | 0.23 (0.004) |
| 10bq | 9b | -(CH2)4- | H | CF2I | 13 | 0.23 (0.004) |
| 10br | 9e | -(CH2)4- | 4-F | CF2Cl | 19 | 0.84 (0.04) |
| 10bs | 9e | -(CH2)4- | 4-F | CF2Br | 32 | 0.45 (0.03) |
| 10bt | 9e | -(CH2)4- | 4-F | CF2I | 60 | 0.15 (0.002) |
| 10bu | 9v | -(CH2)2- | 2-(OCF3) | CF2Cl | 81 | >20a |
| 10bv | 9v | -(CH2)2- | 2-(OCF3) | CF2Br | 40 | 0.70a (0.002) |
| 10bw | 9v | -(CH2)2- | 2-(OCF3) | CF2I | 50 | 1.88 (0.04) |
