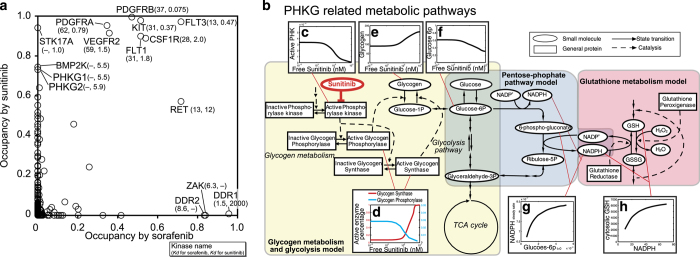
Figure 1.
In silico simulations indicate that off-target inhibition of phosphorylase kinase gamma 1/2 by sunitinib decreases cellular glutathione levels. (a) Comparison of the kinase occupancy profiles of sunitinib and sorafenib. Kinase occupancy was estimated from the mean plasma unbound concentration and Kd value for each drug. (b) Schematic diagram depicting metabolic pathways of glycogen metabolism created from previous reports and knowledge-based information. Phosphorylase kinase (PHK) activates glycogen phosphorylase, an enzyme involved in glycogen catabolism. Glycogen metabolism is linked to glycolysis via glucose-6-phosphate (G6P), which is also shared by the pentose–phosphate pathway. The pentose–phosphate pathway is adjacent to the system regulating glutathione redox balance via production of nicotinamide adenine dinucleotide phosphate (NADPH). (c–f) The effect of sunitinib on the metabolites of glycogen metabolism and glycolysis are simulated. Sunitinib inhibits PHK activity (c) and glycogen phosphorylase activity (d), and increases glycogen synthase activity (d), leading to glycogen accumulation (e). Finally, sunitinib administration decreases G6P levels (f). (g) The effect of the sunitinib-induced lower levels of G6P on NADPH levels in the pentose–phosphate pathway is simulated. This simulation indicates that lower levels of G6P decrease NADPH levels. (h) The effect of lower levels of NADPH (induced by sunitinib) on glutathione (GSH) levels in the glutathione metabolism pathway. This simulation indicates that lower NADPH levels lead to lower GSH levels.