Abstract
It has been reported that both SCID mice and SCID patients poorly mobilize hematopoietic stem/progenitor cells (HSPCs) in response to granulocyte colony-stimulating factor (G-CSF). This defect has been proposed to result from a lack of naturally occurring IgM immunoglobulins to trigger activation of the complement cascade (ComC) and release of C5 cleavage fragments crucial in the mobilization process. However, SCID individuals also have T-cell deficiency, and T cells have been shown to modulate trafficking of HSPCs. To learn more about the role of T lymphocytes, we performed mobilization studies in T-lymphocyte-deficient nude mice and found that these mice respond poorly to G-CSF and zymosan but are normal mobilizers in response to AMD3100. Since nude mice have normal levels of IgM immunoglobulins in peripheral blood and may activate the ComC, we focused on the potential involvement of Gr1+ granulocytes and monocytes, which show defective maturation in these animals. Using a nude mouse mobilization model, we found further support for the proposition that proper function of Gr1+ cells is crucial for optimal mobilization of HSPCs.
Keywords: Complement, Granulocyte degranulation, Stem cell mobilization
Introduction
Hematopoietic stem/progenitor cells (HSPCs) are retained in the bone marrow (BM) microenvironment, and their retention is a result of the interaction of receptors expressed on the surface of HSPCs and their corresponding ligands present in BM stem cell niches1,5. The most important retention signals involve interaction of the α-chemokine receptor CXCR4 and the α1β4 integrin receptor very late antigen 4 (VLA-4), both of which are present in HSPCs. Their respective ligands, stromal-derived growth factor 1 (SDF-1) and vascular adhesion molecule 1 (VCAM-1; also known as CD106), are expressed in the BM microenvironment1,6.
Egress of HSPCs from BM into peripheral blood (PB) is observed in several physiological and pathological situations4,7–14. The number of HSPCs circulating in PB:
-
i)
follows circadian rhythm changes7
-
ii)
is enhanced in several situations related to tissue and organ injuries and strenuous exercise8,10
-
iii)
increases up to 100-fold after administration of promobilizing drugs, such as cytokine granulocyte colony-stimulating factor (G-CSF), or certain small molecules that affect retention of HSPCs in BM niches, such as AMD3100 (also known as plerixafor), which is a C-X-C chemokine receptor type 4 (CXCR4) receptor antagonist11,12.
The cytokine G-CSF is currently the most frequently employed clinical drug and efficiently mobilizes HSPCs after a few consecutive daily injections. AMD3100 is also currently employed in the clinic. A significant level of mobilization may also be achieved within 1 h in experimental animals after injection of the polysaccharide zymosan11,13,14.
Evidence has accumulated that activation of the complement cascade (ComC) is most likely a crucial step in initiating the cascade of events in the mobilization process4,15–17. ComC is activated in all of the above-mentioned situations in which mobilization of HSPCs is observed, including circadian rhythms, infections, tissue and organ injuries, and after administration of pro-mobilizing drugs (e.g., G-CSF or AMD3100 in humans and zymosan in mice)7,12. An important role for ComC in HSPC mobilization is demonstrated by the fact that mice deficient in the fifth component of ComC (C5) are poor mobilizers16.
Some of the first changes observed in the BM during mobilization are induction of a proteolytic micro-environment1,18 and the release of lipolytic enzymes19, both of which affect retention of HSPCs in BM niches. The secretion of these enzymes in the BM microenvironment is stimulated by C5 cleavage fragments, C5a and desArgC5a, known also in the literature as anaphylatoxins. To induce mobilization, C5 cleavage fragments require granulocytes, which are the source of proteolytic and lipolytic enzymes involved in the release of HSPCs from BM niches19,21. Moreover, C5a and desArgC5a in PB chemoattract granulocytes and monocytes from BM, which, as the first cells to cross the BM–PB endothelial barrier, pave the way for HSPCs to follow4,16,22. Confirming a crucial role of granulocytes and mono-cytes, these granulocyte differentiation antigen (Gr-1+) cells11,23,24, as in ComC activation25,28, were found to be indispensable for the mobilization process.
Prompted by observations that T cells have been shown to modulate the trafficking of HSPCs29,31, we performed studies in T-cell-deficient nude mice to learn more about the role of the immune system in mobilization of HSPCs. We observed that, despite having normal immunoglobulin M (IgM) levels and being able to activate ComC, nude mice are poor mobilizers in response to G-CSF and zymosan. We suggest that this defect in nude mice is not due to a lack of T cells but on a defect of granulocytes and monocytes, which results in impaired migration in response to C5 cleavage fragments and defective degranulation of granulocytes. Therefore, we have provided further support for the notion that the proper functioning of granulocytes and monocytes is indispensable for the mobilization of HSPCs and that their defect is responsible for poor mobilization observed here in nude mice.
Materials and Methods
Animals
Pathogen-free, 4- to 6-week-old BALB/c (nude mouse background control) and nude female mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) at least 2 weeks before the experiments. Animal studies were approved by the Animal Care and Use Committee of the University of Louisville (Louisville, KY, USA).
Murine Bone Marrow-Derived Mononuclear Cells (BM-MNCs)
BM-MNCs were obtained by flushing femurs and tibias of pathogen-free, 6- to 8-week-old mice. The cells were lysed with BD Pharm Lyse buffer (BD Biosciences, San Jose, CA, USA) to remove red blood cells (RBCs), washed, and resuspended in appropriate media for further analysis11,16,17,21.
Sorting of Gr-1+ Cells and Monocytes
Cells were isolated from the BM of BALB/c and nude female mice. Briefly, the BM was flushed from tibias and femurs, and the population of total nucleated cells was obtained after lysis of RBCs using 1× BD Pharm Lyse buffer. Cells were subsequently stained with antibodies: phycoerythrin (PE)–anti-CD11b (clone M1/70; BD Biosciences Pharmingen), allophycocyanin (APC)–Cy7–anti-Ly6G (clone 1A8; BioLegend, San Diego, CA, USA), and Alexa Fluor 488–anti-Ly6C (clone HK1.4; BioLegend) for 30 min in medium containing 2% fetal bovine serum (FBS; Seradigm; VWR Life Science, Radnor, PA, USA). Cells were then washed, resuspended in Roswell Park Memorial Institute (RPMI)-1640 medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), and sorted using a Moflo XDP cell sorter (Beckman Coulter, Indianapolis, IN, USA) as populations of neutrophils (Ly6G+/CD11b+) and monocytes (Ly6G–/CD11b+/Ly6Chigh)19.
Mobilization
Mice were injected subcutaneously (SC) with 100 μg/kg of G-CSF (Amgen, Thousand Oaks, CA, USA) daily for 6 days. For zymosan (Sigma-Aldrich, St. Louis, MO, USA) mobilization, mice were injected intravenously (IV) with 0.5 mg/mouse. For AMD3100 (Sigma-Aldrich) mobilization, animals received this compound at a dose of 5 mg/kg SC. At 6 h after the last G-CSF administration or at 1 h after zymosan and AMD3100 injection, mice were bled from the retro-orbital plexus for plasma and hematology analysis, and PB was obtained from the vena cava with a 25-gauge needle and 1-ml syringe containing 50 μl of 100 mM ethylenediaminetetraacetic acid (EDTA; Quality Biological Inc., Gaithersburg, MD, USA). MNCs were obtained by hypotonic lysis of RBCs in BD Pharm Lyse buffer as previously described11,15–17,19.
Fluorescence-Activated Cell Sorting (FACS) Analysis
Staining was performed in RPMI-1640 medium containing 2% FBS. All monoclonal antibodies (mAbs) were added at saturating concentrations, and the cells were incubated for 30 min on ice, washed twice, and analyzed with an LSR II flow cytometer (BD Biosciences). The following mAbs were used to perform staining of Lin−/Sca-1+/c-Kit+ (SKL) cells and Lin–/Sca-1+/CD45+ [hematopoietic stem cells (HSCs)]: FITC–anti-CD117 (also known as c-Kit; clone 2B8; BioLegend) and PE-Cy5-anti-mouse Ly6 A/E (also known as Sca-1; clone D7; eBioscience, San Diego, CA, USA). All anti-mouse lineage markers (Lin) were purchased from BD Biosciences: anti-CD45R/ B220 (clone RA3-6B2), anti-Ter-119 (clone TER-119), anti-CD11b (clone M1/70), anti-T-cell receptor β (clone H57-597), anti-Gr-1 (clone RB6-8C5), anti-TCRγδ (clone GL3), and anti-CD45 (clone 30-F11), and conjugated with PE as previously described11,15–17,19.
Evaluation of HSPC Mobilization
The following formula was used for evaluation of circulating colony-forming unit-granulocyte/macrophage (CFU-GM) and Lin–/Sca-1+/c-Kit+ (SKL) cells: [number of white blood cells (WBCs) × number of CFU-GM colo-nies]/number of WBCs plated = number of CFU-GM per microliter of PB; and (number of WBCs × number of SKL cells)/number of gated WBCs = number of SKL cells per microliter of PB11,15–17,19.
PB Parameter Counts
To obtain leukocyte and RBC counts, 50 μl of PB was taken from the retro-orbital plexus of the mice and collected into microvette EDTA-coated tubes (Sarstedt Inc., Newton, NC, USA). Samples were analyzed within 2 h of collection on a HemaVet 950 (Drew Scientific Inc., Waterbury, CT, USA)11,15,16,19.
Activation of the ComC
Normal plasma from BALB/c or nude mice was exposed to purified Gr-1+ cells (105 cells) from BALB/c or nude mice at 37°C for 30 min. Samples were stored at −80°C until use. All Abs, standard proteins, enzyme reagents, and substrate solutions were purchased from BD Biosciences. Assays were performed according to standard protocols. Briefly, plasma samples were incubated in triplicate wells of microtiter plates, which had been precoated with antigen-specific (C5a) capture Abs. Repeated washing and aspiration were conducted before each incubation step to remove unbound materials from the assay plates. This step was followed by incubation with specific biotin-labeled capture Abs and enzyme reagent. Substrate solution was added to the wells of the microtiter plates, and color developed in proportion to the amount of antigen bound in the initial step of the assay. The color development was stopped with the addition of an acid solution, and the intensity of color was read using a microtiter plate spectrophotometer (Coulter DTX 880 Multimode Detector; Beckman Coulter, Brea, CA, USA). The concentrations of complement C5a in plasma samples were determined by interpolation from individual standard curves composed of purified human C5a. Purified rat anti-mouse C5a (clone I52-1486) was used as the capture Ab (2 μg/ml, overnight at 4°C). Biotin-labeled rat anti-mouse C5a (clone I52-278) was used as the detection Ab (2 μg/ml, 2 h at room temperature). Plasma samples were used at a 1:10 dilution for C5a detection15.
Clonogenic In Vitro Assay
RBCs from PB were lysed with BD Pharm Lyse buffer. Nucleated cells were subsequently washed twice and used for CFU-GM colonies. Briefly, cells were resuspended in human methylcellulose base media provided by the manufacturer (R&D Systems, Minneapolis, MN, USA), supplemented with 25 ng/ml recombinant murine granulocyte macrophage colony-stimulating factor (mGM-CSF; Millipore, Billerica, MA, USA) and 10 ng/ml recombinant murine interleukin-3 (mIL-3; Millipore). Cultures were incubated for 7 days, at which time they were counted for the number of CFU-GM colonies under an inverted microscope (Olympus CK40; Olympus, Shinjuku, Tokyo, Japan). To evaluate the number of clonogenic progenitor cells, BM-MNCs were supplemented with erythropoietin (5 U/ml; Stemcell Technologies, Vancouver, BC, Canada) plus stem cell factor (SCF; 5 ng/ml; R&D Systems) and resuspended in methylcellulose base medium (for determining the number of burst-forming units-erythroid) (R&D Systems), supplemented with thrombopoietin (100 ng/ml; Gibco Thermo Fisher Scientific, Waltham, MA, USA) plus mIL-3 (10 ng/ml; ProSpec-Tany Technogene Ltd., East Brunswick, NJ, USA), and the resuspended plasma clots were used for determining the number of CFU megakaryocytes. The CFU-GM assay was performed as above. Cultures were incubated for 7 days (37°C, 95% humidity, and 5% CO2), at which time they were counted under an inverted microscope for the number of each type of colony, as previously described17,19.
Degranulation Assay
Sorted Gr-1+ cells from BALB/c and nude mice resus-pended in RPMI-1640 medium plus 0.5% bovine serum albumin (BSA; Sigma-Aldrich) (2 million cells per 400 μl of medium) were incubated overnight at 37°C. Subsequently, cells were stimulated by adding C5a (140 ng/ml), desArgC5a (140 ng/ml), or medium alone as control, and for myeloperoxidase (MPO), elastase, and phospholipase C (PLC) activity, cells were incubated for 5 h at 37°C. For MPO and elastase activity, cells were also stimulated with G-CSF (100 ng/ml) and AMD3100 (3 μM). Cells were centrifuged and conditioned medium (CM) was collected. MPO activity was determined using 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich) as described. Briefly, 20-μl samples were combined with 100 μl of 3,3′,5,5′-tetramethylbenzidine substrate solution, and the plate was incubated at 37°C for 20 min. The reaction was stopped by adding 50 μl of 1 M H2SO4 (Mallinckrodt, Dublin, Ireland), and absorption was measured at 450 nm to estimate MPO activity. Elastase activity was measured using the EnzChek Elastase Assay Kit according to the manufacturer's instructions (Life Technologies, Carlsbad, CA, USA). Briefly, 100 μl of fresh sample was incubated with 100 μl of substrate solution (with 25 μg/ml DQ elastin) for up to 4 h at room temperature in the dark, and the resulting fluorescence was recorded at 515-nm emission following 505-nm excitation. PLC activity was measured using the Amplex Red Phospholipase C Assay Kit (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocol, by measuring the fluorescence using excitation in the range of 530–560 nm and emission detection at ∼590 nm. All activity assays were performed in triplicate in 96-well microtiter plates and analyzed with a Beckman Coulter DTX 880 Multimode Detector. Results are shown as a percentage of control samples (p ≤0.05)19.
Transwell Migration Assay
BM-MNCs, BM-derived Gr-1+ cells, and monocytes from BALB/c and nude mice were resuspended in assay medium (RPMI-1640 plus 0.5% BSA). Assay medium (650 μl) without cells, containing SDF-1 (100 ng/ml; Pepro Tech, Rocky Hill, NJ, USA), sphingosine 1-phosphate (S1P; 0.1 μM; Cayman Chemical Company, Ann Arbor, MI, USA), and also C5a (140 ng/ml; BD Biosciences) or desArgC5a (140 ng/ml) purchased from Calbiochem (La Jolla, CA, USA) for Gr-1+ cells and monocytes, was added to the lower chambers of a Costar Transwell 24-well plate (Corning Costar, Cambridge, MA, USA). Aliquots of cell suspension (1 × 106 cells per 100 μl) were loaded onto the upper chambers with 5-μm-pore filters and then incubated for 3 h (37°C, 5% CO2). Gr-1+ cells and monocytes from the lower chambers were harvested and counted by FACS analysis. Briefly, the cells were gated according to their forward scatter (FSC) and side scatter (SSC) parameters and counted during a 30-s acquisition at a high flow rate. After chemotaxis, BM-MNCs from the lower chamber were resuspended in human methylcellulose base medium provided by the manufacturer (R&D Systems), supplemented with murine GM-CSF (25 ng/ml) and IL-3 (10 ng/ml), for determining the number of CFU-GM colonies. Cultures were incubated for 7 days (37°C, 95% humidity, and 5% CO2), at which time they were counted under an inverted microscope for the number of colonies16,17,19.
Statistical Analysis
Arithmetic means and standard deviations were calculated using Instat 1.14 software (Graphpad, San Diego, CA, USA). Statistical significance was defined as p ≤0.05. Data were analyzed using Student's t-test for unpaired samples.
Results
Nude Mice Are Poor Mobilizers in Response to G-CSF and Zymosan but Normal Mobilizers in Response to AMD3100
To learn more about the role of the immune system in the mobilization of HSPCs, we performed studies in T-cell-deficient nude mice. Both nude and background control BALB/c mice were mobilized by employing G-CSF, zymosan, and AMD3100 (Fig. 1). As reported in our previous work, while G-CSF and zymosan induce mobilization by activating ComC, AMD3100 mainly blocks the SDF-1–CXCR4 interaction between the BM niche and HSPCs32,34. Evaluating the number of mobilized nucleated cells, the number of mobilized SKL and CD34– SKL cells, and the number of mobilized CFU-GM circulating in PB, we found that nude mice are poor mobilizers in response to G-CSF and zymosan but normal mobilizers in response to AMD3100.
Figure 1.
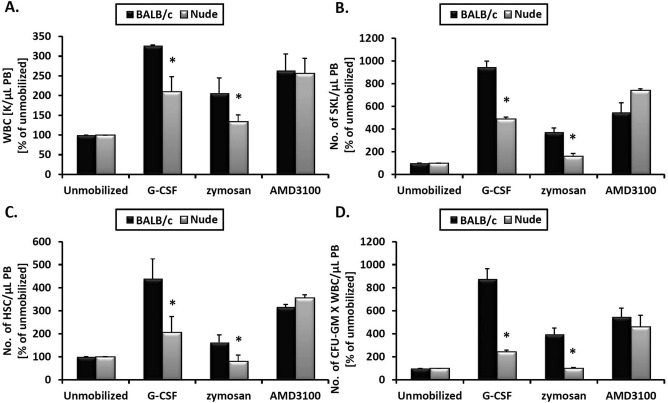
Nude mice are poor mobilizers in response to G-CSF and zymosan but normal mobilizers in response to AMD3100. Nude and BALB/c (control) mice were mobilized with six doses of granulocyte colony-stimulating factor (G-CSF) administered once a day, and 6 h after the last dose the peripheral blood was analyzed. Mice were injected with zymosan or AMD3100, and 1 h later the peripheral blood was analyzed. At the times indicated above, peripheral blood (PB) samples were collected and analyzed for the numbers of white blood cells (WBCs) (A), number (No.) of (Lin−/Sca-1+/c-kit+) (SKL) cells (B), hematopoietic stem cells (HSCs; Lin−/Sca-1+/CD45+) (C), and colony-forming unit-granulocyte/macrophage (CFU-GM) clonogenic progenitors (D). Experiments were performed three times with four mice per group. *p ≤0.05, differences between BALB/c and nude mice. Results are shown as percent of values of unmobilized mice.
These observations prompted us to focus on the possible mechanisms responsible for these differences. Since nude mice have a normal level of naturally occurring IgM antibodies, which trigger activation of the ComC35,36, we focused on additional mechanisms related to mobilization of HSPCs, such as the involvement of Gr-1+ cells in these animals (neutrophils and monocytes). In support of this involvement, nude mice have a defect in maturation of granulocytes and show a resulting compensatory increase in the number of clonogenic CFU-GM37. As shown in Figure 2, we confirmed an increase in the number of CFU-GM in the BM of nude mice.
Figure 2.
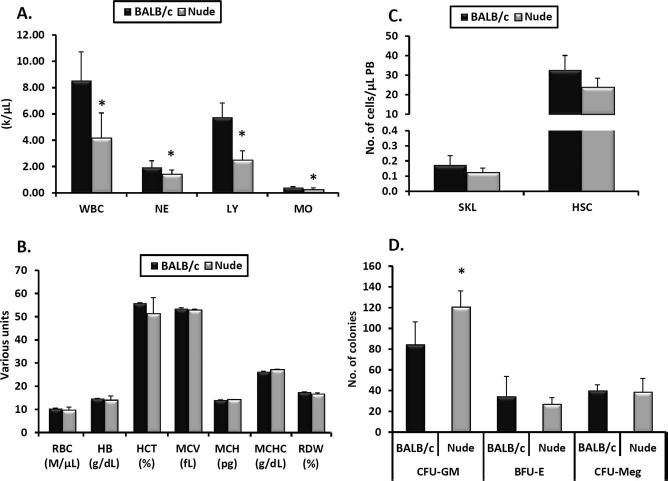
Hematological parameters in nude mice compared with BALB/c animals. Peripheral blood (PB) parameters were evaluated using a HemaVet 950FS analyzer, and nude mice had less white blood cells (WBCs), neutrophils (NEs), lymphocytes (LYs), and monocytes (MOs) (A). Compared with BALB/c (control) mice, nude mice had normal numbers of RBCs, hemoglobin content (HB), hematocrit (HCT), mean volume of erythrocytes (MCV), mean content of hemoglobin (MCH), mean concentration of hemoglobin in erythrocytes (MCHC), and red cell distribution width (RDW) (B). Under steady-state conditions, there were also no differences between nude and BALB/c mice in the numbers of Lin−/Sca-1+/c-kit+ (SKL) cells and hematopoietic stem cells (HSCs) circulating in PB (C). Bone marrow (BM) of nude and BALB/c mice was also isolated and evaluated for the numbers of colony-forming unit-granulocyte/macrophage (CFU-GM), erythroid progenitor cell (BFU-E), and CFU megakaryocyte (CFU-Meg) clonogenic progenitors in in vitro assays, in which nude mice showed higher levels of CFU-GM clonogenic progenitors (D). Data represent an average of at least eight mice tested per experimental group (*p ≤0.05).
Chemotactic Responsiveness of BM-MNCs to Sphingosine-1-Phosphate, SDF-1, C5a, and desArgC5a Gradients
To address the responsiveness of BM cells isolated from nude and control BALB/c mice to chemoattractants that play a role in the mobilization process, we employed Transwell migration assays.
We first observed that BM-MNCs and CFU-GM progenitors show similar chemotactic responses to S1P and SDF-1 gradients (Fig. 3A). Next, since neutrophils and monocytes are the first cells that egress from BM into PB, we studied the responsiveness of FACS-sorted neutrophils (Fig. 3B) and monocytes (Fig. 3C) to C5a and desArgC5 and found that both types of cells purified from nude mice BM show a decrease in chemotactic response to C5 cleavage fragments compared with control animals. This is an important observation, as it has been reported that both C5a and desArgC5a are crucial for the egress of HSPCs into PB and the permeabilization of the blood–BM endothelial barrier16,19–22.
Figure 3.
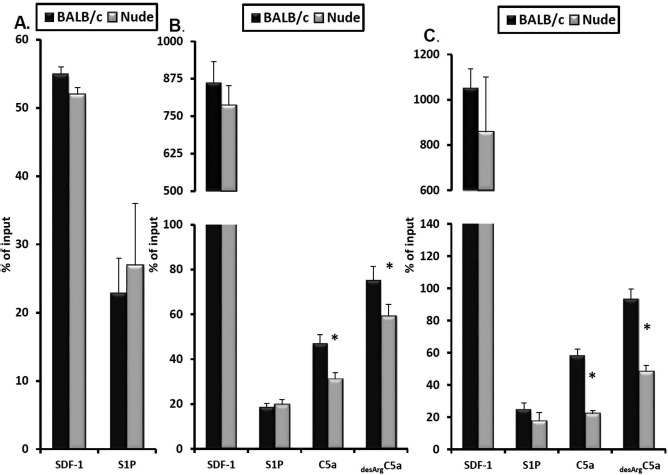
Chemotactic responsiveness of bone marrow-derived mononuclear cells (BM-MNCs) to SDF-1 and S1P gradients and of Gr-1+ cells and monocytes to SDF-1, S1P, and promobilizing C5a and desArgC5a chemoattractant gradients. The chemotactic responsiveness of murine clonogenic colony-forming unit-granulocyte/macrophage (CFU-GM) progenitors to stromal cell-derived factor 1 (SDF-1) and sphingosine 1-phosphate (S1P) gradients (A). Gr-1+ cells (B) and monocytes (C) were employed for chemotaxis assays in response to S1P, SDF-1, complement component 5a (C5a), and desArgC5a gradients using the Transwell system. The experiment was repeated twice, and the data were combined. Results are shown as the percentage of input which represents 5% of the insert (*p ≤0.05).
The Responsiveness of Gr-1+ Cells to Degranulating Agents in Nude and Normal Mice
Gr-1+ granulocytes/monocytes were isolated from the BM of nude and control BALB/c mice and analyzed in a degranulation assay. As degranulating stimulators, we employed G-CSF, AMD3100, C5a, and desArgC5a (Fig. 4). We found that sorted cells from nude mice showed a decrease in the release of elastase and MPO into conditioned media after stimulation by G-CSF and C5 cleavage fragments (Fig. 4A and B). At the same time, no differences were observed after exposure to AMD3100. Similarly, after stimulation by C5a and desArgC5a, Gr-1+ cells from nude mice showed a decrease in the release of a lipolytic enzyme involved in the disintegration of membrane lipid rafts—PLC-β2 (Fig. 4C).
Figure 4.
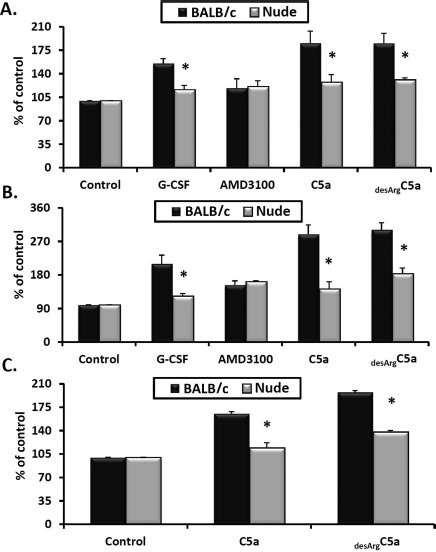
Analysis of nude and BALB/c Gr-1+ cells in degranulation assays. Gr-1+ cells (granulocytes and monocytes) were isolated from the bone marrow (BM) of nude and BALB/c (control) mice and stimulated with medium alone or with G-CSF, AMD3100, C5a, or desArgC5a. After stimulation, conditioned media were analyzed in degranulation assays by measuring elastase activity (A), myeloperoxidase (MPO) activity (B), and phospholipase C activity (C). The results are combined from three independent experiments and show changes as a percentage of the control. Statistical differences refer to the differences between BALB/c and nude mice after stimulation (*p ≤0.05).
Gr-1+ Cells From Nude Mice Show a Defect in the Activation of Complement
Activation of the ComC plays a crucial role in the egress of HSPCs from BM into PB, and there are several factors released by Gr-1+ cells that, in addition to proteolytic enzymes, reactive oxygen species (ROS), and secreted danger-associated molecular pattern molecules (DAMPs), may activate the ComC. To address how effective Gr-1+ cells isolated from nude mice are in activating the ComC, we purified these cells from BM and added them into ice-cold tubes of plasma freshly drawn from BALB/c animals. As shown in Figure 5, in contrast to Gr-1+ cells sorted from BALB/c animals, granulocytes from nude mice show a defect in ComC activation in our assay. In the reverse experiment, we found that plasma drawn from nude mice is somewhat hypersensitive to ComC activation under steady-state conditions, and again BALB/c mouse-derived Gr-1+ cells were able to activate plasma drawn from nude mice more efficiently than Gr-1+ cells isolated from nude animals (Fig. 5).
Figure 5.
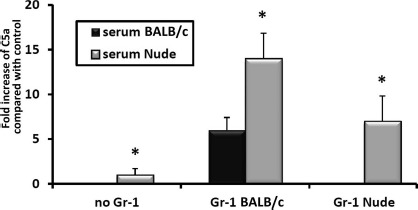
Analysis of Gr-1+ cells isolated from the bone marrow of nude and BALB/c mice for their ability to activate complement. Gr-1+ cells (granulocytes and monocytes) were isolated from bone marrow (BM) of nude and BALB/c mice and exposed to the plasma of BALB/c or nude mice. Complement activation was measured by enzyme-linked immunosorbent assay (ELISA) for C5a. Values are the fold increase of C5a compared with control plasma (*p ≤0.05).
Discussion
The salient observation of our work is that defective mobilization in T-cell-deficient nude mice is related to the defective promobilizing effects of Gr-1+ cells, which additionally provides further evidence for the pivotal role of granulocytes and monocytes in the mobilization process11,23,24. This observation was also supported by employing a different in vivo model than in our previous observation that T lymphocytes are not directly involved in regulating the egress of HSPCs from BM into PB29.
Despite significant progress in the field, the mobilization of HSPCs is still not fully understood, and a significant number of patients, particularly those with previous histories of chemotherapy, are deemed poor mobilizers38,39. Several host-related factors have been described that promote mobilization, including i) release of proteases in the BM microenvironment by activated myeloid cells, which perturb the integrity of the SDF-1–CXCR4 and VCAM-1–VLA-4 retention axes40,41; ii) release of PLC-β2, which perturbs lipid raft assembly on the surface of HSPCs19; iii) downregulation of SDF-1 expression in stem cell niches42,43; iv) upregulation of serpin family protease inhi bitors in the BM microenvironment44,45; and v) downregulation of heme oxygenase 1 (HO-1) activity in HSPCs46.
Moreover, accumulating evidence demonstrates that mobilization of HSPCs is regulated by several components of innate immunity, including naturally occurring IgM antibodies, the ComC, and Gr-1+ granulocytes/monocytes11,25–28. Specifically, we reported that promobilizing agents expose neoepitopes in the BM micro environment that bind naturally occurring IgM Abs, and this leads to activation of the ComC in BM during mobilization4. By contrast, the polysaccharide zymosan may directly activate the ComC in the factor B- and D-mediated, IgM-independent alternative ComC activation pathway11. The ComC is also activated, although less efficiently, by administration of the CXCR4-blocking agent AMD310011,12. The pivotal role of ComC activation and granulocyte activation and egress was reported by us to be perturbed in C5-deficient mice16 and mice that have a defect in activation of the lectin– mannose pathway of ComC activation47 (manuscript in preparation). Similarly, mice that are IgM deficient and Gr-1+ granulocyte deficient are also poor mobilizers4. These observations demonstrate the most likely sequence of events that trigger the mobilization process, including IgM binding to neoepitopes, activation of the ComC, C5 cleavage fragment-mediated degranulation of neutrophils, and egress of Gr-1+ cells from BM to PB as a means to facilitate the subsequent egress of HSPCs.
Our results presented here confirm that nude mice have a defect in the maturation of granulocytes and monocytes37. Moreover, we suggest for the first time that this defect affects several steps in the mobilization of HSPCs. First, Gr-1+ cells from nude mice, when added to BALB/c mouse serum, did not activate the ComC. This defect, however, may depend on the sensitivity of the assay employed. Interestingly, in the reverse ComC activation experiment, we found that plasma in nude mice seem to be hypersensitive to the activation of ComC after exposure to Gr-1+ cells isolated from BALB/c mice. This difference in ComC activation could reflect a kind of compensatory effect in immunodeficient nude mice. Next, we found that Gr-1+ cells from nude mice showed defective degranulation in response to G-CSF, C5a, and desArgC5a. Specifically, they release less elastase, MPO, and PLC-β2 than Gr-1+ cells from control BALB/c animals. Interestingly, at the same time, we did not observe any significant differences in secretion between Gr-1+ cells isolated from nude and BALB/c mice after exposure to AMD3100. Finally, both granulocytes and monocytes purified by FACS showed an impaired chemotactic response to C5a and desArgC5a gradients. This finding is important because, as mentioned above, these cells are the first to egress from BM into PB and are involved in the permeabilization of the blood–BM endothelial barrier for HSPCs. In toto, all these steps involving Gr-1+ cells, which are required for optimal mobilization, are defective in nude mice and explain their poor mobilization status.
In conclusion, we have provided further support for the notion that proper function of Gr-1+ cells is indispensable for the mobilization of HSPCs and that the defects observed in mice with a nude background are responsible for impaired mobilization. We also provided an explanation for the differences in mobilization of HSPCs after exposure to G-CSF and zymosan compared with administration of AMD3100. Analyzing potential differences in the response of Gr-1+ cells to mobilizing agents, the most crucial step that was perturbed in Gr-1+ cells from nude mice was their defective degranulation and release of proteolytic enzymes, which are required to attenuate retention of HSPCs in BM. Since AMD3100, by blocking the CXCR receptor, already attenuates SDF-1–CXCR4-mediated retention, nude mice mobilize to a similar degree as BALB/c mice. In addition, as we observed here, enhanced ComC activation in nude mice is most likely a compensatory immune surveillance mechanism and is most likely sufficient after administration of AMD3100 to facilitate egress of HSPCs from BM into PB.
Acknowledgments
This study was supported by NIH grants R01 CA106281 and R01 DK074720, and the Stella and Henry Hoenig Endowment and Harmonia NCN grant UMO-2014/14/M/NZ3/00475 to M.Z.R., and by NIH grants P20 GM103492, P01 HL078825, and AHA13SDG14560005 to M.W. Dr. Abdel-Latif is supported by the University of Kentucky Clinical and Translational Science Pilot Award (UL1TR000117), the UK COBRE Early Career Program (P20 GM103527), and NIH grant R56 HL124266. The authors declare no conflicts of interest.
References
- 1.Lévesque J.P., Helwani F.M., Winkler I.G.. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia 2010; 24(12): 1979–92. [DOI] [PubMed] [Google Scholar]
- 2.Bonig H., Papayannopoulou T.. Hematopoietic stem cell mobilization: Updated conceptual renditions. Leukemia 2013; 27(1): 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapidot T., Kollet O.. The brain-bone-blood triad: Traffic lights for stem-cell homing and mobilization. Hematology Am Soc Hematol Educ Program 2010; 2010: 1–6. [DOI] [PubMed] [Google Scholar]
- 4.Ratajczak M.Z., Kim C.H., Wojakowski W., Janowska-Wieczorek A., Kucia M., Ratajczak J.. Innate immunity as orchestrator of stem cell mobilization. Leukemia 2010; 24(10): 1667–75. [DOI] [PubMed] [Google Scholar]
- 5.Doan P.L., Chute J.P.. The vascular niche: Home for normal and malignant hematopoietic stem cells. Leukemia 2012; 26(1): 54–62. [DOI] [PubMed] [Google Scholar]
- 6.Lapidot T., Dar A., Kollet O.. How do stem cells find their way home? Blood 2005; 106(6): 1901–10. [DOI] [PubMed] [Google Scholar]
- 7.Méndez-Ferrer S., Chow A., Merad M., Frenette P.S.. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009; 16(4): 235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Möbius-Winkler S., Hilberg T., Menzel K., Golla E., Burman A., Schuler G., Adams V.. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J Appl Physiol. 2009; 107(6): 1943–50. [DOI] [PubMed] [Google Scholar]
- 9.Wojakowski W., Tendera M., Kucia M., Zuba-Surma E., Paczkowska E., Ciosek J., Hałasa M., Król M., Kazmierski M., Buszman P., Ochała A., Ratajczak J., Machaliński B., Ratajczak M.Z.. Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. J Am Coll Cardiol. 2009; 53(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paczkowska E., Kucia M., Koziarska D., Halasa M., Safranow K., Masiuk M., Karbicka A., Nowik M., Nowacki P., Ratajczak M.Z., Machalinski B.. Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke 2009; 40(4): 1237–44. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.M., Wysoczynski M., Liu R., Shin D.M., Kucia M., Botto M., Ratajczak J., Ratajczak M.Z.. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia 2010;24(3): 573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slavin S., Mumcuoglu M., Landsberg-Weisz A., Kedar E.. The use of recombinant cytokines for enhancing immunohematopoietic reconstitution following bone marrow transplantation. I. Effects of in vitro culturing with IL3 and GM-CSF on human and mouse bone marrow cells purged with mafosfamide (ASTA-Z). Bone Marrow Transplant. 1989; 4(5): 459–64. [PubMed] [Google Scholar]
- 13.Sweeney E.A., Priestley G.V., Nakamoto B., Collins R.G., Beaudet A.L., Papayannopoulou T.. Mobilization of stem/progenitor cells by sulfated polysaccharides does not require selectin presence. Proc Natl Acad Sci USA 2000; 97(12): 6544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweeney E.A., Lortat-Jacob H., Priestley G.V., Nakamoto B., Papayannopoulou T.. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: Involvement in mobilization of stem/progenitor cells. Blood 2002; 99(1): 44–51. [DOI] [PubMed] [Google Scholar]
- 15.Borkowska S., Suszynska M., Mierzejewska K., Ismail A., Budkowska M., Salata D., Dolegowska B., Kucia M., Ratajczak J., Ratajczak M.Z.. Novel evidence that crosstalk between the complement, coagulation, and fibrin-olysis proteolytic cascades is involved in mobilization of hematopoietic stem/progenitor cells (HSPCs). Leukemia 2014; 28(11): 2148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H.M., Wu W., Wysoczynski M., Liu R., Zuba-Surma E.K., Kucia M., Ratajczak J., Ratajczak M.Z.. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia 2009; 23(11): 2052–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratajczak M.Z., Lee H., Wysoczynski M., Wan W., Marlicz W., Laughlin M.J., Kucia M., Janowska-Wieczorek A., Ratajczak J.. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia 2010; 24(5): 976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lévesque J.P., Liu F., Simmons P.J., Betsuyaku T., Senior R.M., Pham C., Link D.C.. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood 2004; 104(1): 65–72. [DOI] [PubMed] [Google Scholar]
- 19.Adamiak M., Poniewierska-Baran A., Borkowska S., Schneider G., Abdelbaset-Ismail A., Suszynska M., Abdel-Latif A., Kucia M., Ratajczak J., Ratajczak M.Z.. Evidence that a lipolytic enzyme-hematopoietic-specific phospholipase C-β2-promotes mobilization of hematopoietic stem cells by decreasing their lipid raft-mediated bone marrow retention and increasing the promobilizing effects of granulocytes. Leukemia 2015; 30(4): 919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratajczak M.Z., Suszynska M.. Emerging strategies to enhance homing and engraftment of hematopoietic stem cells. Stem Cell Rev. 2016; 12(1): 121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C.H., Wu W., Wysoczynski M., Abdel-Latif A., Sunkara M., Morris A., Kucia M., Ratajczak J., Ratajczak M.Z.. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: A novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia 2012; 26(1): 106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jalili A., Shirvaikar N., Marquez-Curtis L., Qiu Y., Korol C., Lee H., Turner A.R., Ratajczak M.Z., Janowska-Wieczorek A.. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: Further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010; 38(4): 321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruijt J.F., Verzaal P., van Os R., de Kruijf E.J., van Schie M.L., Mantovani A., Vecchi A., Lindley I.J., Willemze R., Starckx S., Opdenakker G., Fibbe W.E.. Neutrophils are indispensable for hematopoietic stem cell mobilization induced by interleukin-8 in mice. Proc Natl Acad Sci USA 2002; 99(9): 6228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christopher M.J., Rao M., Liu F., Woloszynek J.R., Link D.C.. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011; 208(2): 251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber-Lang M., Younkin E.M., Sarma J.V., Riedemann N., McGuire S.R., Lu K.T., Kunkel R., Younger J.G., Zetoune F.S., Ward P.A.. Generation of C5a by phagocytic cells. Am J Pathol. 2002; 161(5): 1849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward P.A.. The dark side of C5a in sepsis. Nat Rev Immunol. 2004; 4(2): 133–42. [DOI] [PubMed] [Google Scholar]
- 27.Huber-Lang M., Sarma J.V., Zetoune F.S., Rittirsch D., Neff T.A., McGuire S.R., Lambris J.D., Warner R.L., Flierl M.A., Hoesel L.M., Gebhard F., Younger J.G., Drouin S.M., Wetsel R.A., Ward P.A.. Generation of C5a in the absence of C3: A new complement activation pathway. Nat Med. 2006; 12(6): 682–7. [DOI] [PubMed] [Google Scholar]
- 28.Asberg A.E., Mollnes T.E., Videm V.. Complement activation by neutrophil granulocytes. Scand J Immunol. 2008; 67(4): 354–61. [DOI] [PubMed] [Google Scholar]
- 29.Reca R., Cramer D., Yan J., Laughlin M.J., Janowska-Wieczorek A., Ratajczak J., Ratajczak M.Z.. A novel role of complement in mobilization: Immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins. Stem Cells 2007; 25(12): 3093–100. [DOI] [PubMed] [Google Scholar]
- 30.Danby R., Rocha V.. Improving engraftment and immune reconstitution in umbilical cord blood transplantation. Front Immunol. 2014; 5: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell A., Malik S., Litzow M., Gastineau D., Roy V., Zubair A.C.. Dual roles of autologous CD8+ T cells in hematopoietic progenitor cell mobilization and engraftment. Transfusion 2015; 55(7): 1758–65. [DOI] [PubMed] [Google Scholar]
- 32.Liles W.C., Broxmeyer H.E., Rodger E., Wood B., Hübel K., Cooper S., Hangoc G., Bridger G.J., Henson G.W., Calandra G., Dale D.C.. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 2003; 102(8): 2728–30. [DOI] [PubMed] [Google Scholar]
- 33.Broxmeyer H.E., Orschell C.M., Clapp D.W., Hangoc G., Cooper S., Plett P.A., Liles W.C., Li X., Graham-Evans B., Campbell T.B., Calandra G., Bridger G., Dale D.C., Srour E.F.. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005; 201(8): 1307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flomenberg N., Devine S.M., Dipersio J.F., Liesveld J.L., McCarty J.M., Rowley S.D., Vesole D.H., Badel K., Calandra G.. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood 2005; 106(5): 1867–74. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M., Austen W.G. Jr, Chiu I., Alicot E.M., Hung R., Ma M., Verna N., Xu M., Hechtman H.B., Moore F.D. Jr, Carroll M.C.. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA 2004; 101(11): 3886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mink J.G., Radl J., van den Berg P., Haaijman J.J., van Zwieten M.J., Benner R.. Serum immunoglobulins in nude mice and their heterozygous littermates during ageing. Immunology 1980; 40(4): 539–45. [PMC free article] [PubMed] [Google Scholar]
- 37.Monteiro J.P., Benjamin A., Costa E.S., Barcinski M.A., Bonomo A.. Normal hematopoiesis is maintained by activated bone marrow CD4+ T cells. Blood 2005; 105(4): 1484–91. [DOI] [PubMed] [Google Scholar]
- 38.Glaspy J.A., Shpall E.J., LeMaistre C.F., Briddell R.A., Menchaca D.M., Turner S.A., Lill M., Chap L., Jones R., Wiers M.D., Sheridan W.P., McNiece I.K.. Peripheral blood progenitor cell mobilization using stem cell factor in combination with filgrastim in breast cancer patients. Blood 1997; 90(8): 2939–51. [PubMed] [Google Scholar]
- 39.Chabannon C., Le Corroller A.G., Viret F., Eillen C., Faucher C., Moatti J.P., Viens P., Vey N., Braud A.C., Novakovitch G., Ladaique P., Stoppa A.M., Camerlo J., Genre D., Maraninchi D., Blaise D.. Cost-effectiveness of repeated aphereses in poor mobilizers undergoing high-dose chemotherapy and autologous hematopoietic cell transplantation. Leukemia 2003; 17(4): 811–3. [DOI] [PubMed] [Google Scholar]
- 40.Lévesque J.P., Hendy J., Takamatsu Y., Simmons P.J., Bendall L.J.. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003; 111(2): 187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lévesque J.P., Hendy J., Winkler I.G., Takamatsu Y., Simmons P.J.. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003; 31(2): 109–17. [DOI] [PubMed] [Google Scholar]
- 42.Jin C., Fu W.X., Xie L.P., Qian X.P., Chen W.F.. SDF-1alpha production is negatively regulated by mouse estrogen enhanced transcript in a mouse thymus epithelial cell line. Cell Immunol. 2003; 223(1): 26–34. [DOI] [PubMed] [Google Scholar]
- 43.Wright N., de Lera T.L., Garcia-Moruja C., Lillo R., García-Sánchez F., Caruz A., Teixidó J.. Transforming growth factor-beta1 down-regulates expression of chemokine stromal cell-derived factor-1: Functional consequences in cell migration and adhesion. Blood 2003; 102(6): 1978–84. [DOI] [PubMed] [Google Scholar]
- 44.Winkler I.G., Levesque J.P.. Mechanisms of hematopoietic stem cell mobilization: When innate immunity assails the cells that make blood and bone. Exp Hematol. 2006; 34(8): 996–1009. [DOI] [PubMed] [Google Scholar]
- 45.van Pel M., van Os R., Velders G.A., Hagoort H., Heegaard P.M., Lindley I.J., Willemze R., Fibbe W.E.. Serpina1 is a potent inhibitor of IL-8-induced hematopoietic stem cell mobilization. Proc Natl Acad Sci USA 2006; 103(5): 1469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wysoczynski M., Ratajczak J., Pedziwiatr D., Rokosh G., Bolli R., Ratajczak M.Z.. Identification of heme oxygenase 1 (HO-1) as a novel negative regulator of mobilization of hematopoietic stem/progenitor cells. Stem Cell Rev. 2015; 11(1): 110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adamiak M., Abdel-Latif A., Ratajczak J., Ratajczak M.Z.. A novel and pivotal role of the mannose-binding lectin (MBL) pathway of complement cascade (ComC) activation in triggering mobilization of hematopoietic stem/progenitor cells (HSPCs). Paper presented at: 57th American Society of Hematology Annual Meeting and Exposition; 2015. Dec 5–8; Orlando, FL.


