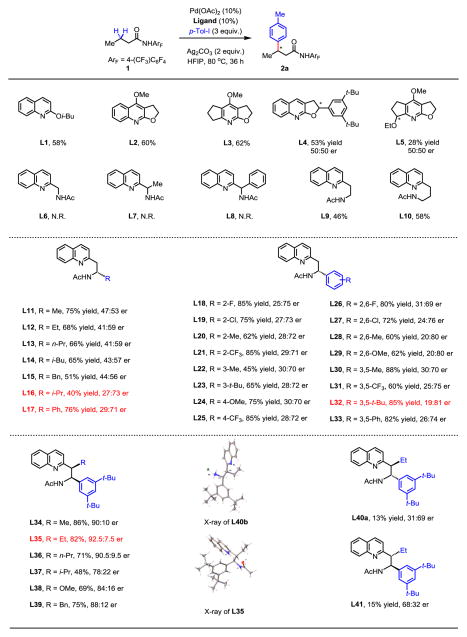
Fig. 2. Ligand development for enantioselective methylene C–H arylation.
The yields were determined by 1H NMR analysis of the crude product using CH2Br2 as an internal standard. Enantiomeric ratios (er) were determined by chiral high-performance liquid chromatography. The absolute configurations of L13, L21, L35 and L40b were determined by X-ray crystallography (see supplementary material). HFIP, hexafluoro-2-propanol; Me, methyl group; Pr, propyl group; Bn, benzyl group; Ph, phenyl group.