Figure 6.
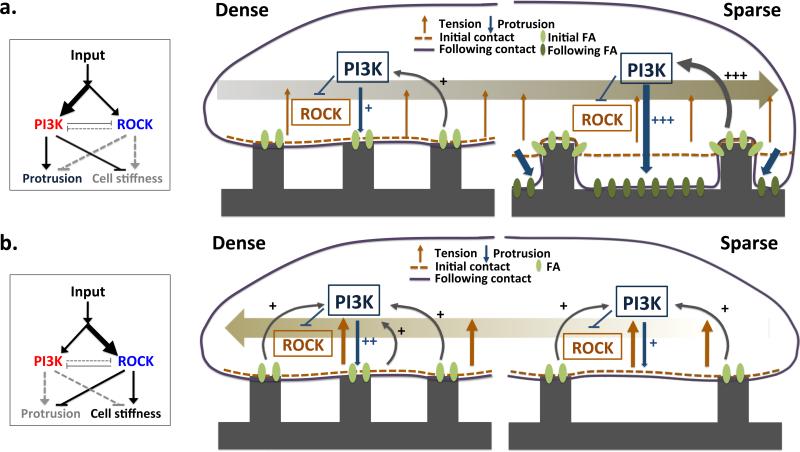
A schematic graphic model of the topotaxis in invasive and non-invasive cells. The directionality of topotactic migration depends on differential conformity of cellular membrane to local topographic structure. The degree of conformity depends on the cell rigidity, which in turn is controlled by the interplay between two ECM activated signaling pathways: PI3K-Akt increasing the degree of conformity and matrix penetration and ROCK-MLCK decreasing the degree of conformity. The balance between the activities of these pathways can be determined by the local ECM signaling input or genetic changes affecting these pathways, and can be modulated by pharmacological perturbations. The relatively higher activity of the PI3K-Akt pathway in the invasive melanoma cells (a) can lead to differential penetration of the matrix and lead to a higher matrix contact in the sparser vs. denser matrix zones, generating bias for migration from the denser to sparser matrix density. In the other hand, at high enough ECM signaling inputs, the relatively higher activity of the ROCK-MLCK pathway in noninvasive melanoma cells limits cell penetration into the matrix, leading to haptotactic migration up the density gradient (b).