Fig. 5. Model of DCV docking and fusion during exocytosis.
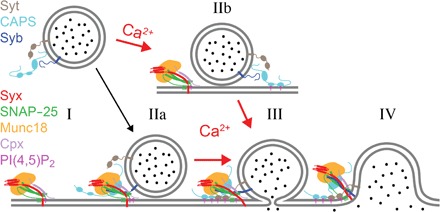
Calcium-dependent release of DCV content requires an acceptor complex consisting of syntaxin-1a, SNAP-25, Munc18, and complexin as well as PI(4,5)P2 in the plasma membrane. The complex interacts with synaptobrevin-2 (syb) and synaptotagmin-1/9 (syt) in the vesicle membrane as well as CAPS that may or may not be vesicle-associated (I). DCVs are able to dock to the complex in the absence of calcium in a SNARE-dependent fashion (IIa). The presence of complexin “clamps” the resulting pre-fusion (trans-SNARE) complex, preventing progression to an open fusion pore, and allows priming of the fusion machinery. Priming depends on CAPS [and/or Munc13 (not shown)] and PI(4,5)P2 and might involve a spatial organization of multiple copies of trans-SNARE complexes and accessory proteins as well as the organization of a specific local nanoscale lipid environment. The primed intermediate state resembles granules in the readily releasable pool in situ. Calcium influx triggers fusion pore opening catalyzed by syts (III) and eventually the collapse of the vesicle membrane into the plasma membrane (IV). Calcium also facilitates CAPS-dependent docking of DCVs to the plasma membrane (IIb). These granules proceed through some intermediates that might resemble the priming steps in the absence of calcium to a fusion pore (III) and eventually to the complete merger of the two membranes (IV).
