Abstract
Background
Calprotectin is a heat stable intracellular protein shed by neutrophils into the intestinal lumen in response to inflammation. Lack of specificity makes its role in the assessment of inflammatory bowel disease uncertain. However, the strength of faecal calprotectin testing may lie in its negative predictive value (NPV) rather than positive predictive value (PPV) of organic intestinal disease.
Objectives
To determine whether a normal faecal calprotectin in new patients with symptoms safely predicts for functional intestinal disease.
Methods
To determine the predictive values of normal and raised faecal calprotectin by retrospective review of outcomes in consecutive primary care referrals into secondary care. Patients aged 16–60 years (mean age 41 years; 70% female patients) with intestinal symptoms were identified. 500 referrals had a normal faecal calprotectin and 130 had a raised result. ‘Fast track’ referrals were excluded. Outcome measures were the NPV of a normal faecal calprotectin and PPV of a raised faecal calprotectin.
Results
Normal faecal calprotectin had an NPV of 0.964 for excluding symptomatic organic intestinal disease. Significant incidental non-intestinal (3.6%) and intestinal (6.4%) diseases were also identified. Mean follow-up was 4.8 years with no diagnostic revisions. In the raised faecal calprotectin cohort, the PPV for organic intestinal disease was 0.7.
Conclusions
A normal faecal calprotectin safely predicts for functional intestinal disease. It may represent a powerful screening tool for excluding organic intestinal disease in primary care. A prospective primary care based study is needed.
Introduction
The diagnosis and management of intestinal disorders represents a major healthcare burden for primary care.1 Much ill health is functional in nature, notably the irritable bowel syndrome (IBS).2 While its management may be challenging, the key is to make a positive diagnosis without unnecessary recourse to invasive and expensive investigations provided by secondary care. The difficulty for primary care is to distinguish these functional illnesses from organic intestinal diseases since there is an overlap of symptomatology between the two populations.3 While functional illness may be treated expectantly, organic intestinal disease often requires prompt investigation and directed treatment. Attempts to apply symptom complexes in differentiating functional from organic diseases are of limited sensitivity and specificity. Blood tests such as the full blood count and C reactive protein are insufficiently discriminating.4
Calprotectin is a heat stable intracellular protein. It is shed by neutrophils during lysosomal degranulation. Neutrophils migrate into the mucosal layer of the small and large intestine as a response to a range of intestinal disease processes that include infection, inflammation and neoplasia. Here some calprotectin will pass into the intestinal lumen and become incorporated into the stool where it can be detected by quantitative assay.5 Based on initial studies on the role of faecal calprotectin, the Department of Gastroenterology at York Hospital started to use this test in the assessment of patients with possible inflammatory bowel disease (IBD).4 At that stage video capsule endoscopy was not widely available and there was a heavy reliance on white cell localisation scanning as well as conventional gastroscopy, colonoscopy and radiology. It rapidly became clear that the faecal calprotectin might have a more powerful role as a negative rather than a positive predictor of organic intestinal disease. Should a normal faecal calprotectin prove to be predictive then it might represent a powerful screening tool for use by primary care allowing for the positive diagnosis of functional disease and so safely reducing the burden of referrals into secondary care.
The aim of this retrospective study was to determine the negative predictive value (NPV) of a normal faecal calprotectin in excluding organic intestinal disease in patients with intestinal symptoms referred unselectively from primary care.
Methods
Recognising its possible utility, between January 2004 and May 2007 the Department of Gastroenterology, composed of four consultants, requested a faecal calprotectin during the investigation of newly referred patients with intestinal symptoms. By agreement with the Department of Chemical Pathology, the faecal calprotectin request was restricted solely to secondary care use. Thus, primary care continued to refer patients with intestinal symptoms unselectively, without recourse to the faecal calprotectin. These patients were then investigated largely without reference to what was for the Department of Gastroenterology an unvalidated test. Conventional modalities of investigation available to a secondary care service were used. Management of patients was a consultant-led service. On request of a faecal calprotectin, a stool sample was delivered by the patient either to the hospital or to their primary care provider. Here, it was forwarded internally to the Department of Chemical Pathology. A polyclonal ELISA (PhiCal test) was used to determine the faecal calprotectin level. The normal cut-off was taken to be 50 µg/g, in line with the manufacturer's guidance. Initially samples were sent on to The Department of Clinical Biochemistry, King's College Hospital, London, for testing. From 2006, the same assay was brought in-house. A quality control sample set at a level of 150 was present in every test batch and with this the coefficient of variation was 5%.
This information was held on a database containing the calprotectin result, the name of the patient, the date of request and the hospital identification number. Ethical approval (REC10/H1302/53) was obtained to perform a retrospective analysis to identify two cohorts of consecutive patients, one with a normal faecal calprotectin and the second with a raised faecal calprotectin. Inclusion criteria were new patient referrals from primary care, aged 16–60 years, with intestinal symptoms. These were defined as any of change of bowel habit, abdominal pain, bloating, mucorrhoea, bleeding, tenesmus or urgency. Patients with fast track colorectal symptoms were excluded from this study.6
The Trust computerised case record system, containing all correspondence, inpatient and outpatient episodes and investigation results, was used to confirm:
-
▶
the patient symptomatology at referral
-
▶
the investigative pathway for the patient
-
▶
the primary intestinal diagnosis
-
▶
incidental diagnoses
-
▶
duration of follow-up
-
▶
revision of original diagnosis
-
▶
the number of inpatient or outpatient contacts.
NPVs and positive predictive values (PPVs) were calculated.
Results
Cohort 1: normal faecal calprotectin
The records of 500 consecutive patients with intestinal symptoms and a normal faecal calprotectin were identified and reviewed. The mean age at referral was 40.5 years and 71% were female patients. In 6.6% of cases a C reactive protein/erythrocyte sedimentation rate, full blood count, albumin, coeliac screen (endomysial antibody/antitissue transglutaminase) or thyroid function test was not requested or could not be located.
Patient symptomatology at referral
The main presenting symptoms are recorded in table 1.
Table 1.
Presenting symptoms at referral
| Presenting symptom | Frequency (%) |
|---|---|
| Pain/discomfort | 84 |
| Diarrhoea | 78 |
| Constipation | 34 |
| Bloat | 72 |
| Bleeding | 12 |
Patient investigations
Forty-three per cent of patients had a full evaluation of the colon by colonoscopy or barium enema and 60% had supportive histology. The type and number of investigations performed and the number of investigations per patient are presented in tables 2 and 3.
Table 2.
Investigative tests performed
| Test performed | N (500) | % |
|---|---|---|
| Rigid sigmoidoscopy | 45 | 9 |
| Flexible sigmoidoscopy | 202 | 40.4 |
| Colonoscopy | 170 | 34 |
| Barium enema | 63 | 12.6 |
| Small bowel follow through | 56 | 11.2 |
| CT scan | 72 | 14.4 |
| MRI scan | 11 | 2.2 |
| Gastroscopy | 176 | 35.2 |
| Ultrasound | 72 | 14.4 |
| Faecal elastase | 34 | 6.8 |
| Anorectal physiology | 8 | 1.6 |
| Lactose hydrogen breath test | 6 | 1.2 |
| Glucose hydrogen breath test | 3 | 0.6 |
| SeHCAT | 5 | 1 |
| Urinary 5-HIAA | 6 | 1.2 |
| White cell localisation | 4 | 0.8 |
| Defaecating proctography | 4 | 0.8 |
| Gut hormones | 3 | 0.6 |
| Meckels scan | 2 | 0.4 |
| Abdominal x-ray | 2 | 0.4 |
| Colonic transit study | 2 | 0.4 |
| RAST test | 1 | 0.2 |
| Intravenous urogram | 1 | 0.2 |
| ERCP | 1 | 0.2 |
| Laparoscopy | 1 | 0.2 |
| Oesophageal physiology | 1 | 0.2 |
| Octreotide scan | 1 | 0.2 |
| Schilling test | 1 | 0.2 |
| Capsule endoscopy | 1 | 0.2 |
| Short synacthen test | 1 | 0.2 |
5-HIAA, 5-hydroxy indoleacetic acid; ERCP, endoscopic retrograde cholangiopancreatography; RAST, radioallergosorbent test; SeHCAT, seleno-25-homo-tauro-cholate scan.
Table 3.
Number of investigations per patient
| Number of investigations | Number of patients (500) |
|---|---|
| 0 | 53 |
| 1 | 175 |
| 2 | 142 |
| 3 | 80 |
| 4 | 39 |
| 5 | 7 |
| 6 | 4 |
Primary intestinal diagnosis
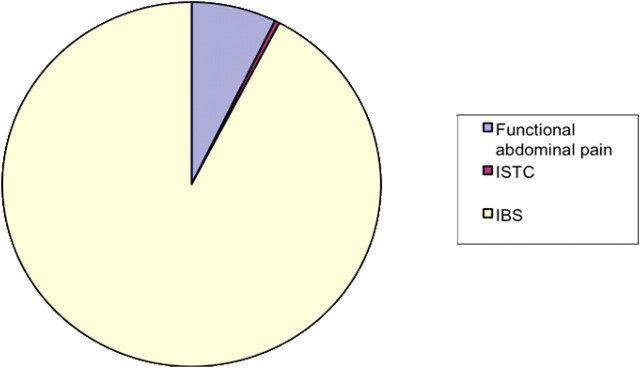
Organic intestinal disease was identified as the primary diagnosis in 18 of the 500 patients referred who had a normal faecal calprotectin (mean age 46.4 vs 40.2 years). The remaining 482 proved to have functional intestinal disease giving an NPV for a normal faecal calprotectin of 0.964 (tables 4 and 5; figures 1 and 2).
Table 4.
Organic intestinal diseases diagnosed with a normal faecal calprotectin
| Organic intestinal diagnosis | N (18) | Age (years) |
|---|---|---|
| Bile salt malabsorption | 4 | 40 |
| 48 | ||
| 48 | ||
| 58 | ||
| Giardiasis | 2 | 32 |
| 50 | ||
| Microscopic colitis | 3 | 47 |
| 58 | ||
| 60 | ||
| Diverticulitis | 2 | 44 |
| 52 | ||
| Crohn's disease | 1 | 22 |
| Coeliac disease | 1 | 51 |
| Chronic pancreatitis | 1 | 31 |
| Thyrotoxicosis | 1 | 56 |
| Small bowel bacterial overgrowth | 1 | 58 |
| Lactose intolerance | 1 | 31 |
| Sorbitol induced diarrhoea | 1 | 50 |
Table 5.
Mean age of patients with functional disease
| Diagnosis | Mean age (years) |
|---|---|
| Functional abdominal pain | 38.6 |
| ISTC | 49.5 |
| IBS | 40.3 |
| D-IBS | 42.1 |
| A-IBS | 38.9 |
| C-IBS | 39.9 |
| M-IBS | 38.4 |
| IBS undefined | 43.7 |
D-IBS, diarrhoea predominant-IBS; A-IBS, alternating-IBS; C-IBS, constipation predominant-IBS; M-IBS, mixed-IBS; ISTC, idiopathic slow transit constipation; IBS, irritable bowel syndrome.
Figure 1.
Functional diagnoses. IBS, irritable bowel syndrome; ISTC, idiopathic slow transit constipation.
Figure 2.
IBS diagnoses. D-IBS, diarrhoea predominant-IBS; A-IBS, alternating-IBS; C-IBS, constipation predominant-IBS; M-IBS, mixed-IBS..
The functional diagnoses are presented below and divided into the IBS subtypes based on the Rome criteria.
The NPV of a normal faecal calprotectin fell in patients over 50 years (0.928) compared with those less than (0.976).
The majority of patients with organic intestinal disease presented with diarrhoea. Nonetheless, the NPV of a normal faecal calprotectin in those with diarrhoea remained high at 0.95 (table 6).
Table 6.
Comparison of symptom complexes between organic and functional intestinal diseases in those with a normal faecal calprotectin
| Functional intestinal disease | Organic disease | |||
|---|---|---|---|---|
| Symptoms | N | % | N | % |
| Pain/discomfort | 406 | 84 | 15 | 83 |
| Diarrhoea | 375 | 78 | 17 | 94 |
| Constipation | 187 | 39 | 1 | 6 |
| Bloat | 347 | 72 | 13 | 72 |
| Bleeding | 67 | 13 | 0 | 0 |
Incidental diagnoses
During the patient investigation, a number of significant diagnoses were made that were incidental to the patients presenting symptomatology. A diagnosis of significant non-intestinal disease was made in 18 (3.6%) patients (table 7) and of significant intestinal disease in 32 (6.4%) (table 8). Minor diverticulosis was judged to be insignificant.
Table 7.
Significant non-intestinal disease in the cohort of patients with normal faecal calprotectin
| Diagnosis | Age (years) |
|---|---|
| Malignancy | |
| Breast cancer | 33 |
| Breast cancer | 42 |
| Renal cell carcinoma | 36 |
| Follicular lymphoma | 42 |
| Follicular lymphoma | 45 |
| Endocrine | |
| Diabetes mellitus | 48 |
| Diabetes mellitus | 59 |
| Hypothyroidism | 24 |
| Hypothyroidism | 58 |
| Hepatological | |
| Hepatitis C | 28 |
| Fatty liver disease | 38 |
| Fatty liver disease | 46 |
| Focal nodular hyperplasia | 32 |
| Liver cyst | 54 |
| Other | |
| Pericardial effusion | 56 |
| Endometriosis | 40 |
| Pulmonary nodules | 38 |
| Urachal remnant | 42 |
Table 8.
Significant incidental intestinal disease in the cohort of patients with normal faecal calprotectin
| Incidental intestinal disease | N | % | Age (years) |
|---|---|---|---|
| Adenomatous polyps | 11 | 35.5 | 46.5 |
| >10 mm | 1 | 3.3 | 48 |
| 5–10 mm tubular adenoma | 1 | 3.2 | 56 |
| <5 mm tubular adenoma | 9 | 29.0 | 45.3 |
| Moderate diverticulosis | 6 | 19.4 | 55.8 |
| Gall stones | 4 | 12.9 | 48 |
| Low vitamin B12 | 4 | 12.9 | 34 |
| Perianal disease | 4 | 12.9 | 36.8 |
| Threadworm | 2 | 6.5 | 37.5 |
| Chronic pancreatitis | 1 | 3.2 | 58 |
Duration of follow-up
Follow-up was for a mean of 4.9 years with only 13 patients lost to follow-up because of relocation outside the York area. There were no deaths in this cohort. Only 11 patients in the cohort were not reviewed by their responsible consultant during this time.
Revision of original diagnosis
During the 4.9-year period of the review, there were no revisions to the diagnosis of intestinal disease in this cohort.
Number of hospital appointments
Reflecting the nature of functional disease, the number of patient contacts during the investigative and management pathway varied considerably (figure 3). For 11% of patients a positive diagnosis of a functional intestinal disorder was made in the outpatient clinic and no additional investigations were performed. For these patients, the mean number of outpatient appointments before discharge was 3.2. By comparison the mean number of outpatient contacts for the remaining patients was 6.9.
Figure 3.
Outpatient activity per patient.
Cohort 2: raised faecal calprotectin
A raised faecal calprotectin was identified in 130 new patients with intestinal symptoms. These patients were identified from the same database as cohort 1 and so give an approximation as to the relative ratio of referred patients with raised to normal faecal calprotectin.
The mean age of such patients was 41 years, with 67% female patients. Mean follow-up was 4.7 years and the mean number of secondary care contacts was 7.9.
A diagnosis of organic disease was made in 91 (70%) patients. A variety of diagnoses were made (table 9).
Table 9.
Diagnoses associated with raised faecal calprotectin
| Diagnosis | Patients (%) | Mean age (SEM) | Mean faecal calprotectin (SEM) |
|---|---|---|---|
| Crohn's disease | 31 | 35 (2) | 503.0 (123.9) |
| Ulcerative colitis | 16.5 | 44.9 (3) | 845.3 (394.4) |
| Indeterminate IBD | 9.9 | 43 (3.5) | 187.7 (76.9) |
| Microscopic colitis | 2.2 | 48 (0) | 116 (23.7) |
| NSAID enteropathy | 14.3 | 43 (13.3) | 196.5 (56.8) |
| Gastroenteritis | 8.8 | 40.6 (3.9) | 458.1 (159.4) |
| Diverticular disease | 2.2 | 42 | 278.5 |
| Bacterial overgrowth | 2.2 | 50 | 386.5 |
| Coeliac disease | 2.2 | 44 | 82.5 |
| Postoperative | 2.2 | 58 | 125 |
| Upper gastrointestinal bleed | 1.1 | 27 | 220 |
| Intussusception | 1.1 | 47 | 193 |
| Rectal polyp | 1.1 | 40 | 65 |
| Alcoholic enteropathy | 1.1 | 56 | 99 |
| Solitary rectal ulcer | 1.1 | 21 | 68 |
| Gastrinoma | 1.1 | 48 | 57 |
| Cholecystitis | 1.1 | 18 | 357 |
| Appendicitis | 1.1 | 48 | 190 |
IBD, inflammatory bowel disease; NSAID, non-steroidal anti-inflammatory drug.
Thirty-nine (30%) patients with a raised faecal calprotectin (mean 164.2) ultimately proved to have a functional intestinal disorder. This gives a PPV of 0.7 in confirming organic intestinal disease.
When the faecal calprotectin was repeated, it was normal in 12 (31%) of the 39 patients resulting in a corrected PPV of 0.77.
A positive faecal calprotectin resulted in extensive intestinal investigation. Among false positives, 75% underwent full colonic evaluation by colonoscopy or barium enema and 58% had small bowel studies either by barium meal and follow through or CT enterography. Capsule endoscopy took place in 12.5% of cases. Supportive histology was obtained in 83% of patients.
Shifting the normal range threshold to 60, 75 and 100 µg/g altered both the NPVs and PPVs (table 10).
Table 10.
PPV and NPV of faecal calprotectin dependent upon the normal range
| Threshold for ‘normal’ faecal calprotectin | NPV | PPV |
|---|---|---|
| <50 | 0.964 | 0.771 |
| <60 | 0.957 | 0.813 |
| <75 | 0.934 | 0.860 |
| <100 | 0.911 | 0.909 |
NPV, negative predictive value; PPV, positive predictive value.
Discussion
The diagnosis of IBS remains a perennial challenge for primary care. Despite attempts to identify symptom complexes that allow for a positive diagnosis, limited sensitivity and specificity mean that a confident diagnosis is often elusive. This places a significant burden upon secondary care gastroenterology services. Ideally, what is needed is a locally available screening test. The test should be sufficiently powerful for a positive diagnosis of functional intestinal disease to be made, providing necessary reassurance to the patient and allowing the primary care physician to focus on symptomatic treatments.7 Such therapy could be delivered locally sparing expensive and unnecessary referral into secondary care. Tibble et al4 first demonstrated the utility of faecal calprotectin as a possible screening test for distinguishing between IBS and IBD. While faecal calprotectin does have a role in the assessment and monitoring of IBD, it is not a sufficiently specific test to permit its positive diagnosis. In a range of disease processes, drug or alcohol induced, infective, inflammatory, ischaemic and malignant, the faecal calprotectin may be elevated. The present study confirms this observation, as 15 diagnoses other than IBD were made during the period of review.
The hypothesis upon which this study was based is that the strength of testing faecal calprotectin lies in the predictive value of a normal result rather than that of an abnormal one. It is postulated that a normal faecal calprotectin allows for the safe and cost-effective local management of IBS within primary care based upon a confident diagnosis. Dolwani et al8 had already demonstrated an NPV of 100% in a small cohort of patients. And in a recent meta-analysis it had been concluded that measuring ‘faecal calprotectin is a useful screening tool for identifying patients who are most likely to need endoscopy for suspected IBD.’9 The authors however expressed reservations about its utility in primary care. To date, no studies have been performed in the primary care setting to refute this.10 11
Presented in this current study are the outcomes of 500 consecutive patients referred from primary care with intestinal symptoms and followed from the point of referral for almost 5 years. This represents a clinically relevant and heterogeneous cohort of patients and for the first time demonstrates both the clinical advantages and difficulties that would arise from using faecal calprotectin as a screening tool. Inflammatory markers and screens for coeliac disease were routinely performed and appropriate investigation and management were consultant led in almost all cases. Patients with fast-track symptoms require definitive investigation and so were excluded from the study. A broad range of patient symptomatology was described, characterised by any change in bowel habit or abdominal pain and bloat and a small but substantial group with what proved to be anal canal bleeding. Within this disparate group of patients a normal faecal calprotectin predicted for functional intestinal disease with 96.4% accuracy. Those aged over 60 years were excluded from the study because of the increase in significant incidental intestinal disease noted with ageing. Potential cost savings are implicit from this study. Sixty-three per cent fewer outpatient management contacts occurred in patients where the diagnosis of a functional disorder was made positively without recourse to investigations. If a normal faecal calprotectin was able to bring a cohort of patients into this management group, considerable savings would accrue. And of those referred, approximately 40% required three consultations or less before discharge. It seems reasonable to conclude that simple medical and dietetic guidance in the setting of a confident diagnosis might be sufficient for many patients. Based on such data, the NHS Centre for Evidence-based Purchasing has recently conducted an economic evaluation of the use of faecal calprotectin.12 The findings from this current study support their projected savings of £13 464 per hypothetical cohort of 1000 patients.
Set against these safety and cost-effectiveness headlines a number of challenges are highlighted by this study. First, within the normal faecal calprotectin cohort, significant incidental non-intestinal and intestinal diseases were identified. Such occurrences would be inevitable in practice and while not directly relevant to the exclusion of intestinal organic disease in patients with symptoms nonetheless result in health benefits. To this end, it is worth noting that the referrals resulted in the diagnosis of five malignancies. Next, it is clear that, with or without a formal diagnosis, the management of functional intestinal symptoms can be very challenging and the data confirm that many patients required prolonged follow-up. Within our unit, this would have involved conventional consultations, close dietetic liaison, IBS nurse specialist input, with access to hypnotherapy, and clinical psychology. Whether primary care can currently offer such a resource is uncertain. Finally, the cost benefit of a normal faecal calprotectin result has to be offset against the unnecessary investigations that arise from false positives. Evidence from the patient record system confirms that patients with a positive faecal calprotectin were more extensively investigated, often with formal small and large bowel investigations, attending hospital on an average approximately eight times compared with five and a half times for those with a normal result. The cost of this would need to be taken into account when considering the merits of a normal faecal calprotectin as a screening test. However, with approximately 39 false positive results for 482 true negatives during the period of review one might infer that only a small proportion of patients would be over investigated compared with the number spared such investigations. The PPV of a raised calprotectin can be improved simply by repeating the test. A subsequent normal result should be interpreted as such. An alternative area to pursue is the cut-off for a normal calprotectin in clinical practice. Increasing the normal range improved the PPV of a raised result but this was offset by a gradual reduction in the NPV of the test. Clearly, maintaining a very high NPV is the key to its value as a screening tool but perhaps increasing the ‘normal’ threshold to 60 would be reasonable from this study.13 14
What is already known on this subject.
-
▶
Faecal calprotectin is a sensitive marker of intestinal inflammation.
-
▶
An increased faecal calprotectin identifies patients who are most likely to have inflammatory bowel disease.
-
▶
The diagnostic accuracy of a raised faecal calprotectin is not known.
What this study adds.
-
▶
Abnormal faecal calprotectin predicts for functional intestinal disease in clinical practice.
How might it impact on clinical practice in the foreseeable future.
-
▶
Invasive investigations may be avoided safely in non-fast track patients with symptoms aged 16–60 years with a normal faecal calprotectin.
-
▶
It has the potential use as a screening tool for primary care physicians.
In conclusion, this retrospective feasibility study demonstrates that a normal faecal calprotectin has the potential to become an integrated screening tool for use in patients with intestinal symptoms. However, the study also exemplifies the challenges that would arise should such a test be adopted. Its negative predictive power makes it a safe test and estimates of cost savings have been projected. However, care must be taken when interpreting these retrospective data. Bias is an inevitable variable; it is unlikely that all patients were recruited and the faecal calprotectin results were not blinded. What now is needed is a well-constructed prospective assessment of its safety and efficacy in a primary care setting. A clinic based qualitative assay is now available to facilitate such a study (PreventID CalDetect).15 16Aside from economic savings, the significant additional benefit is the promise of accelerated diagnosis of those patients hidden among the majority with IBS who have IBD.
Acknowledgments
Dr Ian Holbrook, Top Grade Biochemist, York Hospital, York Teaching Hospital NHS Foundation Trust; Dr Deborah Phillips, Research Advisor, North and East Yorkshire NHS R&D Alliance Learning and Research Centre, York Teaching Hospital NHS Foundation Trust; Dr Victoria Allgar, Statistician, Hull York Medical School, York.
Footnotes
Competing interests: None.
Ethical approval: The study was approved by the National Research Ethics Service, Bradford REC number: 10/H1302/53
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.National Institute for Health and Clinical Excellence. Irritable Bowel Syndrome: Costing Report. London: NICE, 2008. http://www.nice.org.uk/nicemedia/pdf/IBSCostingReport.pdf (accessed 2011). [Google Scholar]
- 2.Thompson WG, Heaton KW, Smyth GT, et al. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut 2000;46:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health and Clinical Excellence. Irritable Bowel Syndrome in Adults: Diagnosis and Management of Irritable Bowel Syndrome in Primary Care. London: NICE, 2008. http://www.nice.org.uk/nicemedia/pdf/IBSFullGuideline.pdf (accessed 2011). [Google Scholar]
- 4.Tibble JA, Sigthorsson G, Foster R, et al. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 2002;123:450–60. [DOI] [PubMed] [Google Scholar]
- 5.Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis 2006;12:524–34. [DOI] [PubMed] [Google Scholar]
- 6.18 Week Commissioning Pathway – Change in Bowel Habit. 2008. http://www.NICE.org.uk/CG027 (accessed 2011).
- 7.Spiller R, Aziz Q, Creed F, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 2007;56:1770–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolwani S, Metzner M, Wassell JJ, et al. Diagnostic accuracy of faecal calprotectin estimation in prediction of abnormal small bowel radiology. Aliment Pharmacol Ther 2004;20:615–21. [DOI] [PubMed] [Google Scholar]
- 9.van Rheenen PF, Van de Vijver E, Fidler V. Faecal Calprotectin for Screening of Patients with Suspected Inflammatory Bowel Disease: Diagnostic Meta-Analysis. BMJ 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jellema P, van Tulder MW, van der Horst HE, et al. Inflammatory bowel disease: a systematic review on the value of diagnostic testing in primary care. Colorectal Dis 2011;13:239–54. [DOI] [PubMed] [Google Scholar]
- 11.Ramadas A, Datta K, Sunderraj L, et al. Faecal calprotectin as part of standardised assessment of suspected irritable bowel syndrome (IBS): a novel investigative algorithm. Gut 2011;60(Suppl 1):A73. [Google Scholar]
- 12.Whitehead SJ, Hutton J. Economic Report: Value of Calprotectin in Screening Out IBS. The York Health Economics Consortium for the Centre for Evidence-Based Purchasing (CEP09041), Feb 2010. http://nhscep.useconnect.co.uk/CEPProducts/Catalogue.aspx?ReportType=Economic+report (accessed 12 Sep 2011).
- 13.Zayyat R, Appleby RN, Logan RPH. Can we improve the negative predictive value of faecal calprotectin for the diagnosis of IBS in primary care? Gut 2011;60 Suppl 1:A49. [Google Scholar]
- 14.Gisbert JP, McNicholl AG. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis 2009;41:56–66. [DOI] [PubMed] [Google Scholar]
- 15.Otten CM, Kok L, Witteman BJ, et al. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med 2008;46:1275–80. [DOI] [PubMed] [Google Scholar]
- 16.Damms A, Bischoff SC. Validation and clinical significance of a new calprotectin rapid test for the diagnosis of gastrointestinal diseases. Int J Colorectal Dis 2008;23:985–92. [DOI] [PubMed] [Google Scholar]