Fig. 4.
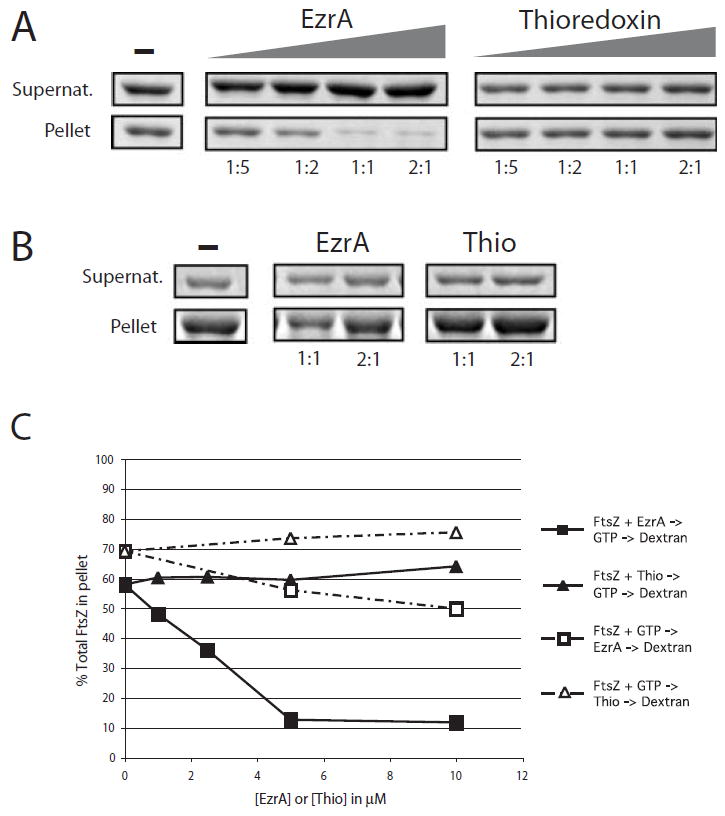
EzrA inhibits sedimentation of FtsZ assembled in vitro. FtsZ is 5 μM in all reactions. The samples were spun at 250 000 g to separate unassembled protein from protofilament bundles, and the relative concentration of protein in the supernatants (unassembled FtsZ) and pellets (assembled FtsZ) was analysed by SDS–PAGE.
A. EzrA and thioredoxin were added to final concentrations of 1, 2.5 μM, 5 μM and 10 μM before the addition of GTP and DEAE-dextran. Left. Increasing concentration of FtsZ in the supernatants and decreasing concentration of FtsZ in the pellets result from the inhibition of FtsZ assembly by purified EzrA. Right. The thioredoxin control protein does not significantly inhibit FtsZ assembly. The ratio of EzrA or thioredoxin to FtsZ is shown under each lane. B. Order of addition. EzrA and thioredoxin were added to the reaction 1 min after the addition of GTP. Ratios of EzrA and thioredoxin to FtsZ are shown below each lane.
C. Plot of percentage of total FtsZ in the pellet. EzrA, squares; thioredoxin, triangles. Closed symbols represent data from the experiment shown in (A). Open symbols represent data from the experiment shown in (B). Differences in the sedimentation efficiency of FtsZ in the absence of EzrA or thioredoxin between the experiments shown in (A) and (B) reflect normal variations between protein preparations.
