Figure 5. Phosphorylation of S183 Occurs during G2-M Phase and Is Not Required for G1-S Cell-Cycle Arrest.
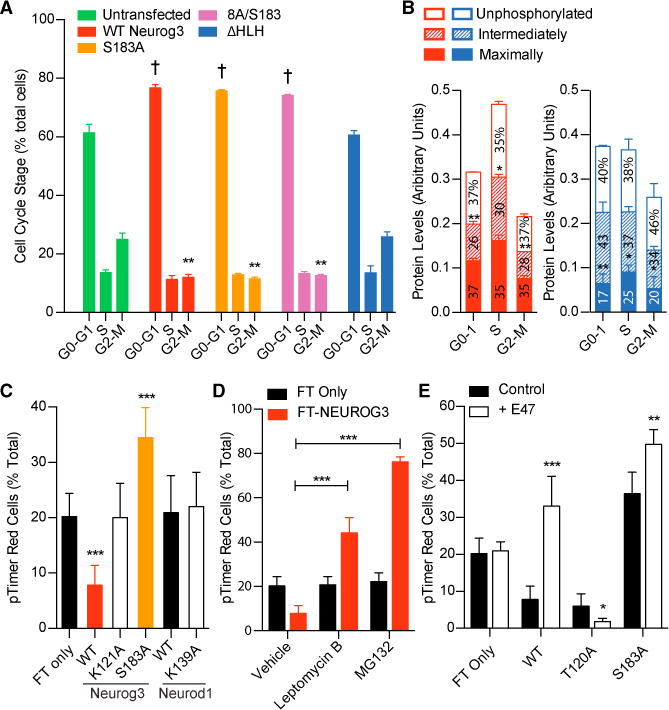
(A) Untransfected HeLa cells and those expressing FLAG-tagged NEUROG3 variants were trypsinized, fixed, and stained with anti-FLAG, anti-pHH3 antibodies, and DAPI, and flow cytometry utilized to determine the number of transfected cells in G0-G1, S, and G2-M. ** or †p < 0.005 compared with untransfected by one-way ANOVA and Dunnett’s post hoc test.
(B) Live HeLa cells expressing FLAG-tagged WT NEUROG3 (red) or NEUROG3ΔHLH (blue) were trypsinized and sorted by FACS into G0-G1, S, and G2-M populations. For each population, the fast-, medium-, and slow-migrating NEUROG3 species (i.e., unphosphorylated, intermediately phosphorylated, and maximally phosphorylated forms, respectively) were quantified by western blotting with anti-FLAG antibody and normalized to β-actin levels. Relative protein levels within each cell-cycle phase that are significantly different from others are indicated by asterisks above that phosphorylation form; *p < 0.05, **p < 0.005 by one-way ANOVA. n = 3.
(C–E) HeLa cells expressing the indicated fluorescence timer (pTimer/FT)-fusion proteins were untreated (C), incubated with leptomycin B or MG132 (D), or cotransfected with E47 (E), prior to flow cytometric analyses to determine the percentage of FT red-containing cells. Significant differences from FT only (C and E) or vehicle (D) are indicated by *p < 0.05, **p < 0.005, and ***p < 0.0005 by one-way ANOVA and Dunnett’s post hoc test. n = 3.
Data are presented as mean ± SEM.
