Abstract
Murine astrovirus (MuAstV) is a recently identified, widespread infection among laboratory mice. Our goal was to determine the duration of MuAstV infection, susceptibility of pups, and efficacy of soiled-bedding sentinels and environmental monitoring. Eight CD1 dams and their litters of 3-d-old pups and 8 CD1 dams and their litters of 13-d-old mice were inoculated orally with MuAstV. Neither dams nor offspring demonstrated any clinical signs, and MuAstV had little to no effect on weight gain in pups. MuAstV RNA was detected in feces from 15 of the 16 dams through postnatal day (PND) 21, and 9 dams were still shedding MuAstV at PND 42. MuAstV RNA was highest in intestines of mice. Low levels of MuAstV RNA were sporadically detected in the spleen, liver, and kidney. MuAstV was detected in 97% of feces from 3- to 9-wk-old mice born to infected dams. Several weanlings became pregnant, and intestines from their pups were MuAstV-negative at PND 0 through 5. Weekly swabs of cages housing MuAstV-infected mice were MuAstV-positive through PND 42. Swabs of the rear exhaust manifold of the ventilated rack were MuAstV-positive at 21 through 56 d after inoculation. In addition, 98% of sentinels that received soiled bedding from dams and their litters and 83% of sentinels that received soiled bedding from weaned mice were MuAstV-positive. Feces from most sentinels exposed to soiled bedding that had been stored for 1, 2 or 3 wk before addition of the sentinels were MuAstV-positive.
Abbreviations: DPI, days after inoculation; EMH, extramedullary hematopoiesis; MLN, mesenteric lymph nodes; MuAstV, murine astrovirus; PND, postnatal day
Murine astrovirus (MuAstV) has recently been detected in commercial and noncommercial mouse colonies, but its effect on murine-based research and biosecurity is largely unknown.10,21,25 Astroviruses were first observed in 1975 by electron microscopy of stool from a child with diarrhea.4 Astroviruses are the second most-common cause of nonbacterial gastroenteritis in children, the elderly, and the immunocompromised. Patients infected with human astroviruses present with diarrhea, vomiting, fever, anorexia, and abdominal pain lasting 2 to 4 d.4 Astroviruses infect a wide range of mammalian species, including cows, sheep, pigs, cats, dogs, bats, and rats, causing self-limiting diarrhea and enteritis or no clinical signs.7 Astrovirus infections in humans cause minimal inflammation, and diarrhea is postulated to be due to disruption of the cellular tight junctions in the intestinal epithelium.20
MuAstV was first observed in 1985 in the gut contents of NMRI and nude mice during an outbreak of diarrhea in nude mice.13 In 2011, as part of a metagenomics study of feces collected from a wild Mus musculus, the first complete genome sequence of a MuAstV was elucidated.22 Portions of 40 other strains of MuAstV from the feces or liver of asymptomatic immunocompetent (BALB/c, C57BL/6, C3H, ICR, CD1) and immunodeficient (NOD-scid, NSG, uPA-NOG, Rag1, Rag2) adult mice have been sequenced.21,25 Attempts to propagate MuAstV in 7 commonly used cell lines have been unsuccessful.10 To understand the role of the immune system in controlling MuAstV infection, one group infected C57BL/6 and Stat1 mice by cohousing them with infected Rag1 mice.25 MuAstV RNA was detected in the feces of all 3 mouse strains on days 2 to 14, with Rag1 mice having the highest level of MuAstV RNA. At day 14, MuAstV RNA was detected in the intestines and mesenteric lymph nodes (MLN) of C57BL/6 mice; in the intestines, MLN, and spleen of Stat1 mice; and in the intestines, MLN, spleen, liver and kidney of Rag1 mice. The authors25 concluded that humoral, cell-mediated and innate immunity are involved in controlling MuAstV infection. Although the C57BL/6, B6.Stat1−/− and B6.Rag−/− mice all lacked clinical signs and pathology, the effect of MuAstV on research models is unknown, and the authors25 suggested that MuAstV might contribute to differences seen between groups of control and experimental mice when they are housed in facilities or obtained from sources with differing MuAstV infection status. Interferon α recently was shown to be important in the clearance of MuAstV, in that IFNαR-deficient mice had sustained shedding of high levels of MuAstV RNA for at least 7 wk, whereas C57BL/6 mice shed lower levels of MuAstV RNA only during the first 2 wk of infection.17 Both C57BL/6 and IFNαR mice infected with MuAstV for 3 d had greater intestinal permeability than uninfected control mice, with IFNαR mice having a greater increase in permeability.17
Few publications with practical information regarding MuAstV infections and their biology in the context of laboratory animal medicine are available, and the potential of MuAstV to confound research results merits investigation. To effectively monitor mouse colonies for MuAstV, the duration of viral shedding and the efficacy of molecular methods to detect MuAstV in feces and in the environment must be known. In addition, whether MuAstV—like human, ovine, bovine, porcine, and canine astroviruses—demonstrates age-dependent infection and disease is unclear.7
To mimic the natural infection of neonates in a endemically infected population, we inoculated MuAstV at 2 critical ages: in 3-d-old mice, when well-characterized murine viruses (mouse hepatitis virus and murine rotavirus) produce their highest viral titers and the greatest pathology,1,24 and in 13-d-old mice, when coprophagy begins.8 We also assessed the ability of soiled-bedding sentinels and environmental monitoring to detect MuAstV.
Materials and Methods
Mice.
Timed pregnant or 4-wk-old female CD1 (CRL:CD1[ICR]) mice were obtained from Charles River (Raleigh, NC). Vendor reports indicated that mice were seronegative for ectromelia virus, Hantaan virus, murine rotavirus, K virus, lactate dehydrogenase-elevating virus, lymphocytic choriomeningitis virus, mouse hepatitis virus, mouse parvovirus, minute virus of mice, murine adenovirus, murine norovirus, mouse thymic virus, polyoma virus, pneumonia virus of mice, reovirus, Sendai virus, and Theiler encephalomyelitis virus and were free of excluded bacterial and parasitic infections at the time of shipment. On arrival, feces from all mice were collected for MuAstV RT-PCR testing. Mice were housed in IVC under positive pressure (ACE MicroVent, Allentown, NJ). Cages containing corncob bedding (Harlan Teklad, Indianapolis, IN), rodent chow (Global 2018S, Harlan Teklad), and nesting material (Cotton square, Ancare, Bellmore, NY) were preassembled and autoclaved. Mice drank hyperchlorinated (4 to 6 ppm) water delivered by water bottle without restriction. The mice were housed and husbanded by using standard biocontainment procedures. The animal room had a negative pressure differential relative to the corridor, a 12:12-h light:dark cycle, 10 to 15 air changes hourly, temperature of 22.2 ± 1.1 °C, and humidity of 50% ± 10%. All animal care and experimental procedures were performed in an AAALAC-accredited animal facility and were approved by the Yale IACUC. All animal care conformed to guidelines in the Guide for the Care and Use of Laboratory Animals,11 and experimental procedures were in accordance with federal policies and guidelines governing the use of vertebrate animals.
Murine astrovirus stocks and inoculation.
In 2014, 162 fecal samples from mice submitted for routine molecular diagnostics were screened for MuAstV RNA by using RT-PCR analysis; a third of the samples (53 of 162) were positive for MuAstV RNA. Fecal homogenates from 6 MuAstV RNA-positive CD1 mice were pooled and clarified by centrifugation. Three female SW mice (CRL:CFW[SW]; age, 6 wk) were obtained from Charles River (Kingston NY), and each mouse was inoculated orally with 20 μL of clarified fecal homogenate. At 5 d after inoculation (DPI), mice were euthanized by carbon dioxide, and 4 sections of the intestine (the upper half of the small intestine, the lower half of the small intestine, the cecum, and the colon) were collected from each mouse. These tissue samples were processed as 10% homogenates in DMEM; RT-PCR analysis indicated that the colonic homogenates had the highest level of MuAstV RNA. Experimental mice in our study were inoculated with pooled colonic homogenates that had been clarified by centrifugation and had been passed through a 0.22-μm filter. This MuAstV stock was negative for nucleic acids from Helicobacter spp., mouse hepatitis virus, mouse parvovirus, murine norovirus, murine rotavirus, reovirus, Theiler encephalomyelitis virus, and Tritrichomonas ssp. according to PCR analysis. A portion of ORF1b and the complete ORF2 region of MuAstV strain Y were sequenced, and the data have been submitted to GenBank (accession no. KX683863). The capsid gene of MuAstV-Y has 90% to 92% nucleotide homology and 92% to 94% amino acid-homology with MuAstV strains STL1, STL2, STL3, STL4, BSRI1, and TF18LM.
RT-PCR analysis for MuAstV.
RNA was extracted from samples by using RNeasy kits (Qiagen, Valencia, CA) according to the manufacturer's instructions. RT- PCR analysis for MuAstV was performed by using the primers MuAstV-BF (5′ GAA TTT GAC TGG ACA CGC TTT GA 3′) and MuAstV-BR (5′ GGT TTA ACC CAC ATG CCA AA 3′),21 iScript One-Step RT-PCR kit with SYBR Green (BioRad, Hercules, CA), and a thermocycler (CFX Connect, BioRad). All assays contained negative and positive controls. The reaction conditions were 10 min at 50 °C; 5 min at 95 °C; and 40 cycles of 10 s at 95 °C, 20 s at 59 °C, and 30 s at 72 °C. The standards used for quantification of MuAstV RNA levels were dilutions of an RT-PCR product (DNA) and encompassed 968 nucleotides of the MuAstV RNA-dependent RNA polymerase. The PCR signal from 1 pg of MuAstV cDNA equaled 1 unit.
Infection of dams with 3- or 13-d-old litters.
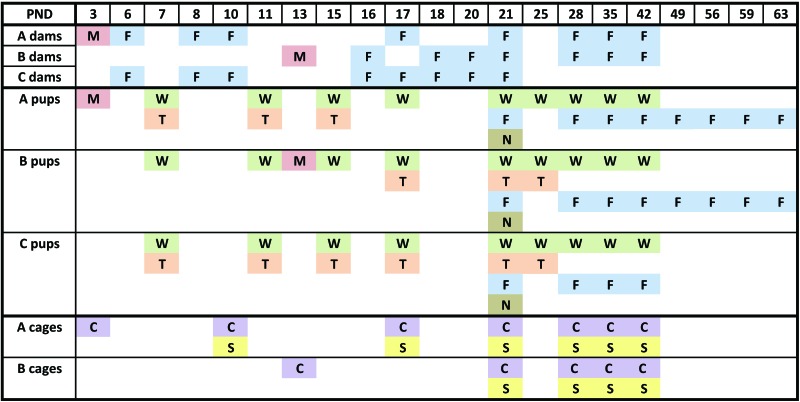
The timeline of the experimental design is shown in Figure 1. A fecal pellet was collected from the anus of each of the 20 timed-pregnant CD1 mice on their arrival. Feces were frozen and stored at –70 °C for subsequent RT-PCR testing to confirm that the mice were negative for MuAstV infection. These pregnant mice were housed singly, and cages were changed weekly. Litters were born over a 3-d period and were allocated to experimental and control groups as follows: day 1, 4 litters to group B and 1 to group C; day 2, 4 litters to group B and 1 to group C; and day 3, 8 litters to group A and 2 to group C. At postnatal day (PND) 3, litters were culled to a maximum of 10 pups. In addition, on PND 3 (group A) and 13 (group B), 8 dams were each inoculated orally with 20 μL of MuAstV-Y stock, and each pup in her litter was inoculated orally with 10 μL of MuAstV-Y stock. The 4 control dams were inoculated orally with 20 μL of DMEM, and each pup in her litter was inoculated orally with 10 μL of DMEM on PND 3 (group C).
Figure 1.
Timeline of MuAstV infection study. C (purple), cage surfaces swabbed for MuAstV RT-PCR analysis; F (blue), feces collected for RT-PCR analysis; M (red), MuAstV inoculation; N (brown), mice weaned; PND, postnatal day of litter; S (yellow), sentinels added to soiled bedding; T (orange), tissues collected for histopathology and MuAstV RT-PCR analysis.
Feces were collected from the anus of group A dams at PND 6, 8, 10, 17, 21, 28, 35, and 42 (3, 5, 7, 14, 18, 25, 32, and 39 DPI) and from the anus of group B dams at PND 16,18, 20, 21, 28, 35, and 42 (3, 5,7, 8, 15, 22 and 29 DPI). Feces were frozen at –70 °C prior to RT-PCR testing for MuAstV RNA. On PND 7, we used a waterproof, permanent marker to mark 4 (2 female, 2 male) mice per litter, and these 80 pups were weighed at PND 7, 11, 15, 17, 21, 25, 28, 35, and 42. Tissues (described following) were collected from 120 pups (60 pups for MuAstV RT-PCR and 60 pups for histopathologic analysis). Specifically, groups of 16 pups (2 per litter) inoculated at PND 3 were euthanized and necropsied at PND 7, 11, and 15 (4, 8, and 12 DPI) for MuAstV RT-PCR and histopathologic analysis. Similarly, groups of 16 pups (2 per litter) inoculated at PND 13 were euthanized and necropsied at PND 17, 21, and 25 (4, 8, and 12 DPI), and groups of 4 control pups were euthanized and necropsied at PND 7, 11, 15, 17, 21, and 25.
Pups were weaned at PND 21 and were housed 4 per cage (group A, 32 pups; group B, 48 pups; and group C, 24 pups). After weaning of their litters, inoculated dams were housed 4 per cage (4 cages). Feces were collected from the anus of all weanlings on PND 21, 28, 35, and 42 and were frozen at –70 °C until MuAstV RT-PCR analysis. For future studies, intestines and sera were collected from all dams and most of the weanlings between PND 42 and 45.
Feces from 8 female weanlings each in groups A and B were collected at PND 49, 56, 59, and 63 to determine the duration of shedding from weanlings; 9 of these 16 weanlings were pregnant, and 4 had delivered litters by 9 wk of age. Intestines from pups from these 4 litters were collected at PND 0 to 5 for MuAstV RT-PCR testing.
Tissue collection and histopathologic analysis.
Mice were euthanized by decapitation (age, 7 d) or carbon dioxide asphyxiation followed by exsanguination by cardiocentesis (age, 10 d or older) and necropsied. Internal organs were examined for gross lesions, and the small and large intestine, pancreas, mesenteric lymph nodes, spleen, liver, and kidneys from each mouse were harvested, immersion-fixed in 10% neutral buffered formalin, trimmed, processed, embedded in paraffin, sectioned, and stained with hematoxylin and eosin by routine methods. Tissues from 8 inoculated mice and 4 control mice were examined at each time point. The slides were examined blind to experimental manipulation (CJB), and tissues were evaluated for histopathologic changes. In addition, portions of intestines, spleens, livers, and kidneys were collected for RT-PCR analysis from another 8 inoculated mice and 4 control mice at each time point.
Soiled-bedding transmission of MuAstV.
At 7 DPI (PND10), the 8 cages of mouse dams and their litters inoculated at PND 3 (group A) were changed. Soiled bedding was collected from each cage; 100 mL of the soiled bedding from each cage was placed in each of 2 cages containing clean bedding, and a 4-wk-old female CD1 mouse was added to each cage to serve as a soiled-bedding sentinel (total of 16 sentinels). The remaining soiled bedding from the 8 cages was mixed, and 100 mL of the mixed bedding was added to each of 6 cages containing clean bedding. These 6 cages were placed on the IVC rack for 1, 2, or 3 wk; a single sentinel CD1 mouse was added to each cage of ‘aged’ soiled bedding at 1, 2, or 3 wk after cages were placed on the rack (2 cages per time point). At 14 and 18 DPI (PND 17 and 21), the 8 cages of mouse dams and their litters inoculated at PND 3 (group A) were changed; 100 mL of soiled bedding from each cage was placed into 2 cages containing clean bedding, and a 4-wk-old female CD1 mouse was added to each cage to serve as a soiled-bedding sentinel (16 sentinels at each time point).
At 8 DPI (PND 21), the 8 cages of mouse dams and their litters inoculated at PND 13 (group B) were changed; 100 mL of soiled bedding from each cage was placed into 2 cages containing clean bedding, and a 4-wk-old female CD1 mouse was added to each cage to serve as a soiled-bedding sentinel (16 sentinels at each time point). The remaining soiled bedding from the 8 cages was mixed, and 100 mL of the mixed bedding was added to each of 6 cages and the cages placed on the IVC rack for 1, 2, or 3 wk. A single 4-wk-old female CD1 sentinel mouse was added to each cage of ‘aged’ soiled bedding at 1, 2 or 3 wk after the cages were placed on the rack (2 cages at each time point). On PND 28, 35, and 42, the 4 cages of inoculated dams and the 16 cages of weanling mice were changed; 100 mL of soiled bedding from each cage was placed into a cage containing clean bedding, and a 4-wk-old female CD1 mouse was added to each cage to serve as a soiled bedding sentinel.
All sentinels were exposed to soiled bedding for 7 d and then moved to new cages with clean bedding for an additional 7 d. For MuAstV RT-PCR analysis, feces were collected from sentinels at 7 and 14 d after exposure to soiled bedding.
Testing cage surfaces and exhaust manifolds of IVC racks for MuAstV.
All sides of the each soiled cage housing MuAstV mice were swabbed at the level of the bedding by using a sterile flocked swab (Puritan, Guilford, ME) weekly starting on the day of inoculation. The exhaust manifolds on the IVC rack were tested by swabbing the upper, middle, and lower sections of the exhaust manifold on the end of the rack and the exhaust manifold on the rear of the rack when the pregnant female mice were placed on the rack (–8 DPI) and at 0, 7, 14, 21, 28, 35, 41, 49, and 55 DPI by using sterile flocked swabs (Puritan).
Statistical analysis.
Two-tailed t tests were performed by using Excel (Microsoft, Redmond, WA). A P value of less than 0.05 denotes statistical significance.
Results
MuAstV infection in dams.
Overall 7 of the 8 dams inoculated on PND 3 of their litter (group A) had detectable levels of MuAstV RNA in their feces through 18 DPI (Table 1). The remaining group A dam yielded only minimal MuAstV RNA in feces at 3 DPI and none at later time points, indicating she did not become infected despite being housed with 3 other dams after her litter was weaned. Fecal MuAstV RNA levels did not differ among group A dams at 3, 5, or 7 DPI. The MuAstV RNA level in feces from group A dams peaked at 18 DPI (Table 1). The number of group A dams with detectable levels of MuAstV RNA in their feces decreased to 4 (57%) by 39 DPI, and the amount of MuAstV detected in the feces of these dams at 39 DPI was less than 0.01% of the peak level (Table 1).
Table 1.
Dam fecal PCR analysis
| Group | DPI 3 PND 6 | DPI 5 PND 8 | DPI 7 PND 10 | DPI 14 PND 17 | DPI 18 PND 21 | DPI 25 PND 28 | DPI 32 PND 35 | DPI 39 PND 42 | |
| A (n= 7)a | % positive | 100 | 100 | 100 | 100 | 100 | 86 | 71 | 57 |
| mean ± 1 SDb | 1618 ± 815 | 894 ± 796c | 1735 ± 1135d | 1596 ± 2349 | 7301 ± 8488 | 3292 ± 5704 | 1317 ± 1953 | 0.58 ± 0.89 |
| DPI 3 PND 16 | DPI 5 PND 18 | DPI 7 PND 20 | DPI 8 PND 21 | DPI 15 PND 28 | DPI 22 PND 35 | DPI 29 PND 42 | ||
| B (n = 8) | % positive | 100 | 100 | 100 | 100 | 100 | 100 | 63 |
| mean ± 1 SD | 63,584 ± 80,902 | 85,233 ± 68,566c | 60,806 ± 34,258d | 10,071 ± 12,320 | 1763 ± 3048 | 724 ± 1192 | 2.13 ± 5.30 |
Data from the dam that did not become infected were not included
Average units (pg. of DNA) per fecal pellet; limit of detection is 0.04 U/fecal pellet
c,d P < 0.05 between values with the same superscripted letter
All 8 dams inoculated on PND 13 of their litter (group B) had detectable levels of MuAstV RNA in their feces through 22 DPI (Table 1). Although mean levels of MuAstV RNA in group B dams did not differ between 3, 5, or 7 DPI, the MuAstV RNA level in group B dams peaked at 5 DPI (Table 1). The number of group B dams with detectable levels of MuAstV RNA in their feces decreased to 5 by 29 DPI, and the amount of MuAstV detected in the feces of these dams at 29 DPI was less than 0.01% of the peak level (Table 1).
Fecal MuAstV RNA levels were higher in group B dams than group A dams at 5 and 7 DPI (P = 0.01 and 0.001, respectively). However, from PND 21 to 42 (18 to 39 DPI for group A and 8 to 29 DPI for group B), fecal MuAstV levels did not differ between dams in groups A and B. MuAstV RNA was not detected in the feces from control mice at any time point. Clinical signs were not observed in any of the dams inoculated with MuAstV.
MuAstV infection in neonatal mice.
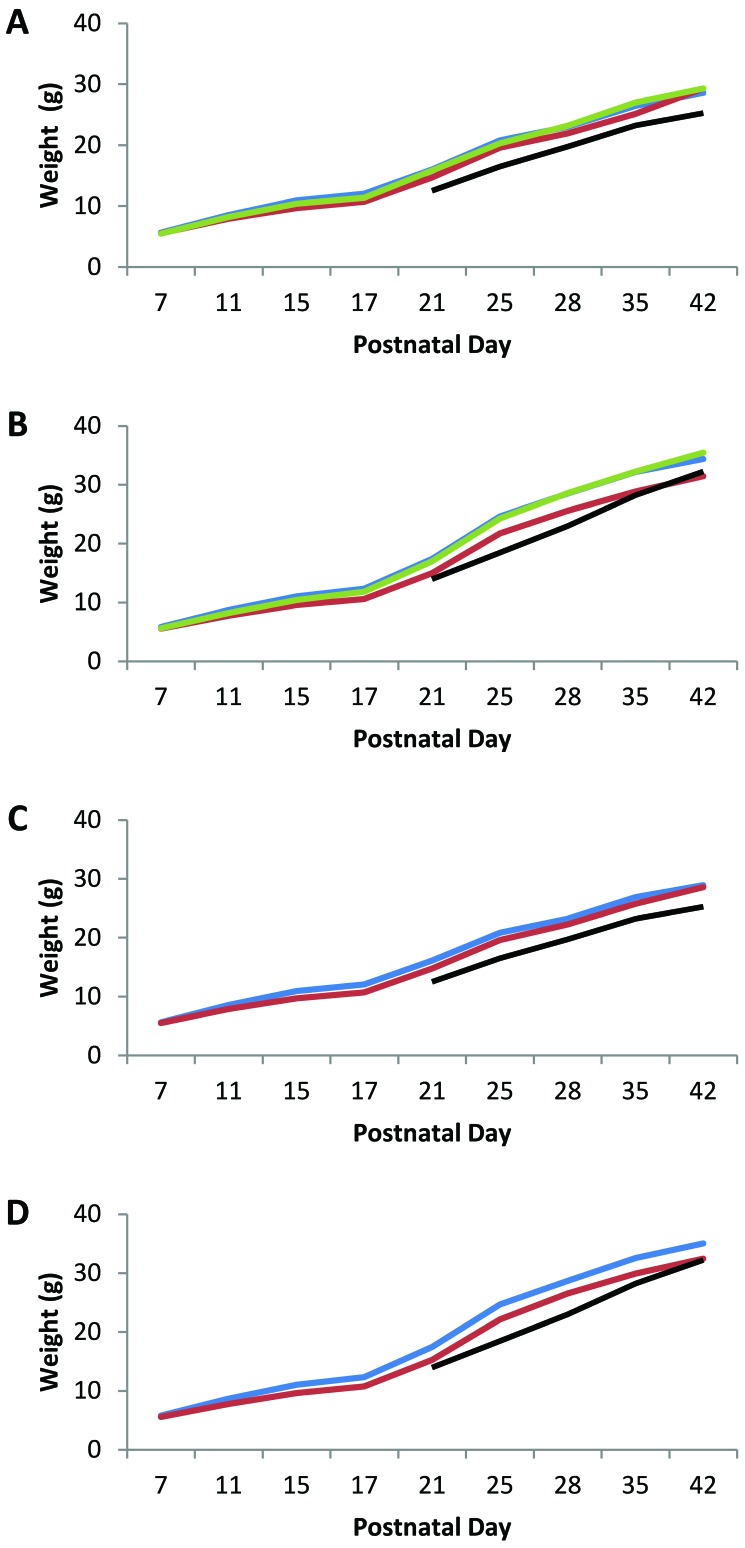
We did not observe clinical signs in any of the MuAstV-infected or control pups. To determine whether MuAstV infection affected weight gain in mice inoculated on PND 3 or 13, neonatal and weaned mice were weighed at PND 7, 11, 15, 17, 21, 25, 28, 35, and 42. Overall, the percentage weight gain over the 5 wk between PND 7 and 42 was 516% for female mice and 593% for male mice. The average weights of mice at 21, 28, 35, and 42 PND in this study were near or exceeded the published mean weight for CD1 mice.5 The average weights did not differ between female group A pups (n = 16) that were inoculated on PND 3 and their sex-matched controls (n = 8; Figure 2 A) nor between male group A pups inoculated on PND 3 (n = 16) and their sex-matched controls (n = 8; Figure 2 B). The average weights of female group B pups (n = 16) inoculated on PND 13 were approximately 7% lower than those of female control mice at PND 15 and 21 (P = 0.02; Figure 2 A). The average weights of male group B pups inoculated on PND 13 (n = 16) were 9% to 12% less than those of male control mice (n = 8) from PND 15 through PND 42 (P = 0.04 to 0.0001; Figure 2 B).
Figure 2.
Average weight of MuAstV-infected and mock-infected control mice. (A) Blue line, group A females inoculated with MuAstV at PND 3; red line, group B females inoculated with MuAstV at PND 13; green line, group C (control) females mock-inoculated at PND 3 or 13; black line, published average weight for weanling female CD1 mice.5 (B) Blue line, group A males inoculated with MuAstV at PND 3; red line, group B males inoculated with MuAstV at PND 13; green line, group C (control) males mock-inoculated at PND 3 or 13; black line, published average weight for weanling male CD1 mice.5 (C) Blue line, females born on day 3; red line, females born on day 1 and 2; black line, published average weight for weanling female CD1 mice.5 (D) Blue line, males born on day 3; red line, males born on day 1 and 2; black line, published average weight for weanling male CD1 mice.5
The litters of mice used in this study were born over 3 d. Analysis of the weight data according to the birthdate of the litters rather than their infection status indicated that a substantial difference in weight gain occurred between litters born on the third day and litters born on the first 2 d. Female pups in litters born on the third day (16 group A mice and 4 control mice) weighed more than those born on the first or second day (16 group B mice and 4 control mice) at all time points, with the most substantial differences in average weight on PND 11 through 25 (6% to 11%; P = 2.5 × 10−3 to 1.4 × 10−7; Figure 2 C). Similarly male pups in litters born on day 3 (16 group A mice and 4 control mice) weighed more than those born on the day 1 or 2 (16 group B mice and 4 control mice) at all time points, with substantial differences in average weight on PND 11 through 42 (7% to 13%; P = 2.6 × 10−3 to 2.6 × 10−10; Figure 2 D). This effect on weight gain occurred before as well as after MuAstV infection and was not due to litter size, which ranged from 11.5 to 11.8 pups per litter on average regardless of birth date. Date of birth apparently had a greater effect on weight gain than did MuAstV infection. The average weight of mice in all groups—regardless of sex, date of birth, or infection status—fell within the normal weight range for CD1 mice;5 consequently these observed differences are clinically irrelevant.
PCR analysis and histopathology of tissues from neonatal and weanling mice.
To investigate the tissue tropism of MuAstV in neonatal mice, 16 pups inoculated on PND 3 (group A) were euthanized at PND 7, 11, and 15 (4, 8, and 12 DPI), 16 progeny mice inoculated on PND 13 (group B) were euthanized at PND 17, 21, and 25 (4, 8, and 12 DPI), and 4 control mice were euthanized at PND 7, 11, 15, 17, 21, and 25. MuAstV RT-PCR analysis was performed on homogenized tissues (Intestines, spleens, livers, and kidneys) from 8 group A and 8 group B mice at each time point (Table 2). Overall, MuAstV RNA was detected in 98% (47 of 48) of intestine samples, with the highest level of MuAstV RNA detected between PND 15 and 21. Trace levels of RNA were detected in 4% (1 of 24) of spleens from group A mice and from 46% (11 of 24) of spleens, 46% (11 of 24) of livers, and 12% (3 of 24) of kidneys from group B mice.
Table 2.
PCR analysis of tissue samples from neonatal and weanling mice
| Group A |
Group B |
||||||
| Tissue (n = 8) | 4 DPI, PND 7 | 8 DPI, PND 11 | 12 DPI, PND 15 | 4 DPI, PND 17 | 8 DPI, PND 21 | 12 DPI, PND 25 | |
| Intestines | no. positive | 8 | 8 | 7 | 8 | 8 | 8 |
| Spleen | no. positive | 0 | 1 | 0 | 8 | 3 | 0 |
| Liver | no. positive | 0 | 1 | 0 | 7 | 4 | 0 |
| Kidney | no. positive | 0 | 0 | 0 | 3 | 0 | 0 |
| Intestines | mean ± 1 SD | 7.0 ± 3.8a | 400 ± 714 | 73,939 ± 31,650 | 319,510 ± 218,635 | 50,922 ± 58,467 | 3543 ± 3119 |
| Spleen | mean ± 1 SD | 0 | 2.5 | 0 | 28 ± 45 | 1.3 ± 1.9 | 0 |
| Liver | mean ± 1 SD | 0 | 28 | 0 | 67 ± 44 | 2.0 ± 3.2 | 0 |
| Kidney | mean ± 1 SD | 0 | 0 | 0 | 9 ± 23 | 0 | 0 |
Average units (pg of DNA) per gram of tissue; limit of detection is 2 U/g tissue
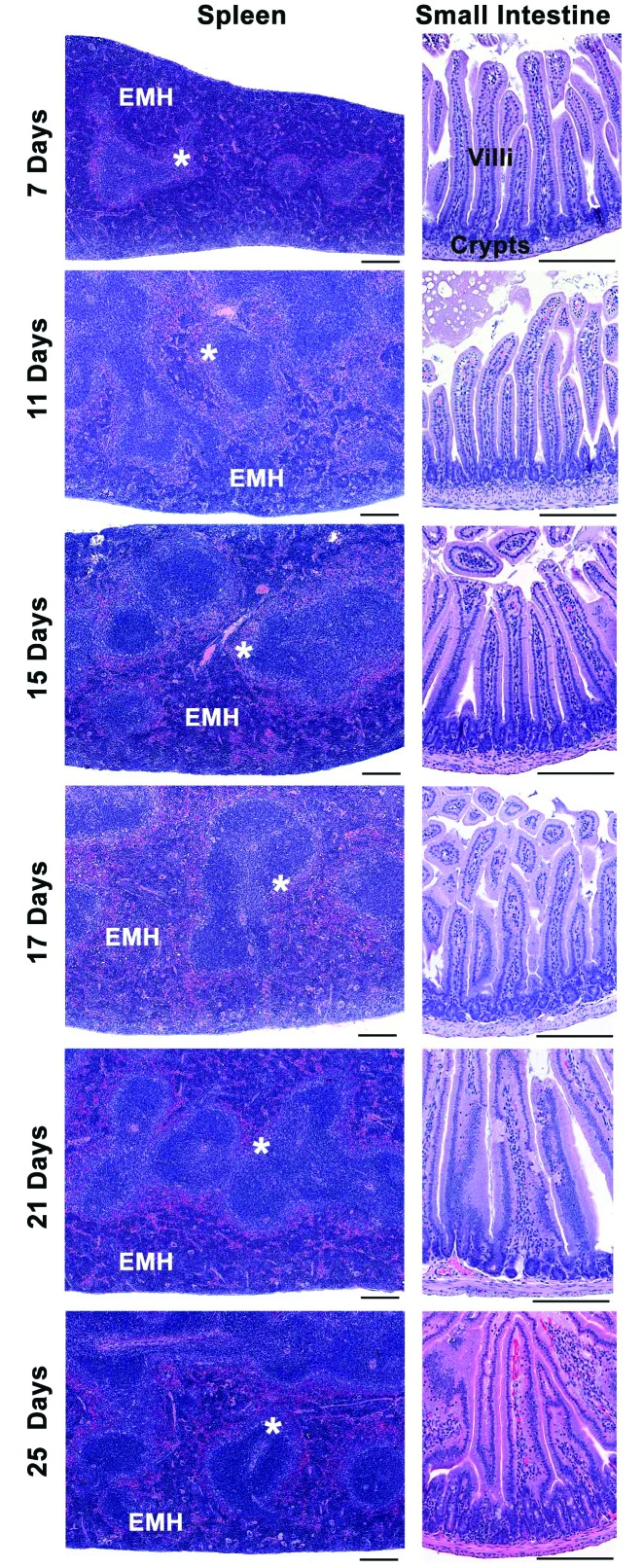
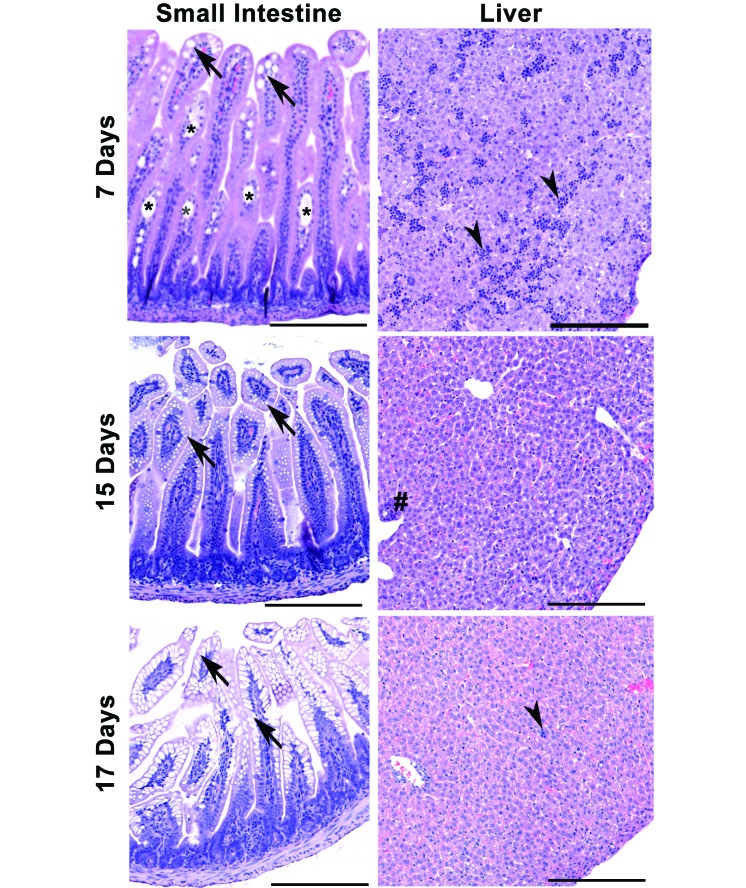
Histopathologic analysis blind to experimental treatment and age was performed on formalin-fixed small and large intestine, spleen, liver, and kidney samples from 8 group A, 8 group B, and 4 control mice at each time point (4, 8, and 12 DPI). We found no gross or microscopic changes suggestive of an infectious disease process in any of the tissues examined from control or MuAstV-infected pups and weanlings. Representative data from small intestine and spleen samples from control and MuAstV-infected mice are shown in Figures 3 and 4 and revealed age-associated developmental changes in both control and MuAstV-infected pups and weanlings, as previously reported.2 The expected shallow crypts of Lieberkühn were most apparent on PND 7 and 11, and small intestinal samples in all mice and at all time points had long villus tips with parallel columnar enterocytes predominantly with homogenous eosinophilic cytoplasm (Figure 3). Other changes included scattered villi with dilated central lacteals and frequent vacuolated enterocytes on PND 7 (Figure 4). By PND 15, many villi had small clear vacuoles, and by PND 17, the vacuoles were large and often filled the villus enterocyte cytoplasm (Figure 4).2 As reported previously,2,15 mouse splenic red pulp is an active site of extramedullary hematopoiesis (EMH) throughout life, and all mice demonstrated robust EMH (Figure 3, left panels), with the majority of red pulp involved in EMH in PND 7 mice and a variable to overall decrease in EMH by PND 25. Splenic white pulp in control and MuAstV-infected mice was unremarkable, with periarteriolar lymphoid sheaths and small, dark lymphoid follicles surrounded by marginal zone (Figure 3). Our mice showed age-related variation in the size of the marginal zone, consistent with the fact that the marginal zone of spleen is not fully formed until 2 to 4 wk of age in mice.16,23 Frequent foci of sinusoidal hematopoietic cells were still present at PND 7; this finding was not unexpected, given that at birth, mouse liver sinusoids contain hematopoietic cells.2 Livers from mice on PND 11 (not shown), 15, and 17 had small scattered foci of periductular or sinusoidal hematopoietic cells (Figure 4).
Figure 3.
Representative sections of spleen and small intestine from control and MuAstV-infected CD1 mice on PND 7 to 25. No histopathologic differences were observed between groups. Left panels: spleen with expected extramedullary hematopoiesis (EMH) in the red pulp and white pulp with the normally variable marginal zone (*) border between the red and white pulp. Right panels: small intestine with expected shallow crypts at PND 7 and 11, and normal crypt and villi present at each time point. Hematoxylin and eosin stain; scale bars, 200 μm.
Figure 4.
Representative images of normal developmental changes in small intestine and liver in both control and MuAstV-infected CD1 mice on PND 7, 15, and 17. Left panels: small intestine with dilated central lacteals (*) and vacuolated villus enterocytes (arrows), with the largest vacuoles visible at PND 17 and absent by PND 21 (data not shown). Right panels: sinusoidal hematopoietic cells (arrowheads) are common in liver at PND 7 but are largely absent by PND 15 onward, except for small clusters around bile ducts (#) or within sinusoids (PND 17, arrow). Hematoxylin and eosin stain; scale bars, 200 μm.
Fecal PCR analysis of weanlings.
To determine the duration of fecal shedding of MuAstV from mice infected as neonates, feces were collected from weanlings at PND 21 through 63. MuAstV RNA was detected through PND 56 in all weanlings inoculated on PND 3 (group A) and in 75% of group A weanlings on PND 59 and 63 (Table 3). MuAstV RNA was detected through PND 63 in all weanlings inoculated on PND 13 (group B; Table 3). The average fecal MuAstV RNA level did not differ significantly between groups or time points. Between PND 47 and 52, 4 of the weanlings delivered litters of mice. Intestines collected from pups from these 4 litters at PND 0 to 5 were all negative for MuAstV RNA. Although the 8-wk-old weanlings were shedding MuAstV RNA and might have been shedding low levels of infectious MuAstV, they did not transmit it to their pups. This finding raises the possibility that pups born to dams chronically infected with MuAstV might be transferred to naïve foster dams during the first 5 d after birth to generate MuAstV-free stocks of mice.
Table 3.
Fecal MuAstV RT-PCR analysis of weanling mice
| Group | PND 21 | PND 28 | PND 35 | PND 42 | PND 49 | PND 56 | PND 59 | PND 63 | |
| A | |||||||||
| % positive (no. positive/no. tested) | 100 (32/32) | 100 (32/32) | 100 (32/32) | 100 (32/32) | 100 (8/8) | 100 (8/8) | 75 (6/8) | 75 (6/8) | |
| mean ± 1 SDa | 31 ± 52 | 136 ± 373 | 142 ± 293 | 586 ± 1592 | 496 ± 853 | ND | 107 ± 240 | ND | |
| B | |||||||||
| % positive (no. positive/no. tested) | 100 (30/30) | 100 (30/30) | 100 (30/30) | 100 (30/30) | 100 (8/8) | 100 (8/8) | 100 (8/8) | 100 (8/8) | |
| mean ± 1 SD | 47 ± 78 | 96 ± 124 | 61 ± 66 | 193 ± 380 | 157 ± 261 | ND | 26 ± 34 | ND |
ND, not done
Average units (pg of DNA) per fecal pellet; limit of detection is 0.04 U per fecal pellet
Detection of MuAstV by using soiled-bedding sentinels.
Feces from almost all of the sentinels exposed to soiled bedding from the group A dams and their litters at 7 and 14 DPI (PND 10 and 17) were positive for MuAstV RNA (Table 4). Feces from all of the sentinels exposed to soiled bedding from the dams and their litters on PND 21 (group A, 18 DPI; group B, 8 DPI) were positive for MuAstV RNA (Table 4). Feces from most of the sentinels exposed to soiled bedding from the 16 cages of weanlings were positive for MuAstV RNA at PND 28,35, and 42 (Table 4). Feces from most of the sentinels exposed to soiled bedding from the cages of dams cohoused after weaning of their litters were positive for MuAstV RNA at PND 28, 35, and 42 (Table 4). Feces from soiled-bedding sentinels were analyzed at both 7 and 14 d after exposure to soiled bedding, and the number of positive sentinels was the same or slightly higher at 14 d after exposure in all groups. Because the sentinels were housed on clean bedding for the second week, this result indicates that the MuAstV RNA detected in the sentinels on 14 d after exposure to bedding represented infectious RNA, not just noninfectious virus ingested from the soiled bedding. The stability of MuAstV in soiled bedding was tested by using soiled bedding collected 1 wk after inoculation and to which sentinels were added 1, 2, or 3 wk later. All 6 sentinels placed on ‘aged’ soiled bedding from group A cages were positive for MuAstV at 7 and 14 d after bedding exposure. Feces from all 6 sentinels placed on aged soiled bedding from group B cages were positive for MuAstV at 7 d after bedding exposure, but feces from only half of the sentinels (1 of 2 at each time point) were positive for MuAstV RNA at 14 d after bedding exposure, implying that only half of the sentinels became infected, whereas the other half ingested MuAstV while housed on soiled bedding but did not become infected.
Table 4.
Fecal MuAstV RT-PCR analysis of soiled-bedding sentinels
| Group | PND 10 | PND 17 | PND 21 | PND 28 | PND 35 | PND 42 |
| A dam and litter | 15/16 | 15/16 | 16/16 | ND | ND | ND |
| B dam and litter | ND | ND | 16/16 | ND | ND | ND |
| A dams | ND | ND | ND | 2/2 | 1/2 | 3/4 |
| B dams | ND | ND | ND | 2/2 | 2/2 | 2/4 |
| A weanlings | ND | ND | ND | 6/8 | 7/8 | 12/16 |
| B weanlings | ND | ND | ND | 6/8 | 8/8 | 12/16 |
ND, not done
Data are given as no. of sentinels positive for MuAstV RNA / no. of sentinels tested 14 d after soiled-bedding exposure
Detection of MuAstV on cage surfaces.
To determine the feasibility of using swabs to sample for MuAstV on cage surfaces, the inner surface of the cage, at the bedding level, was swabbed, and the presence of MuAstV RNA was detected by using RT-PCR analysis. All cages were negative for MuAstV RNA prior to inoculation of the mice. However, 7 of the 8 cages housing group A dams and their litters were positive for MuAstV at 7, 14, and 18 DPI (PND 10, 17, and 21), and all 8 cages that had housed group B dams and their litters were positive for MuAstV at 8 DPI (PND 21). In addition, 7 of the 8 cages that had housed group A weanlings, all 8 cages that had housed group B weanlings, and all 4 cages that housed dams after mice were weaned were positive for MuAstV RNA at PND 28, 35, and 41 (group A, 25 to 38 DPI; group B, 15 to 28 DPI).
Detection of MuAstV on the exhaust manifolds of IVC racks.
To determine the feasibility of using swabs to sample for MuAstV on the exhaust manifold surfaces of the IVC, 4 sites on the exhaust manifolds (upper end, middle end, lower end, and rear) were sampled at –8, 0, 7, 14, 21, 28, 35, 41, 49, and 55 DPI. All samples were negative for MuAstV RNA at –8 through 14 DPI. Between 21 and 55 DPI, 33% of the upper swabs, 50% of both the middle and lower swabs, and 100% of the rear swabs were positive for MuAstV RNA. Therefore, the rear exhaust manifold of the IVC is the preferable sampling site, and the accumulation of a detectable amount of MuAstV on the exhaust manifold surface took more than 2 wk.
Discussion
Murine astrovirus caused no clinical disease in CD1 pups, weanlings, and dams. Our findings are in agreement with a previous study in adult C57BL/6J, B6.Rag1−/−, and B6.Stat1−/− mice, in which MuAstV infection caused no overt illness.25 Although the average weight of female and male mice infected with MuAstV at 13 d of age was lower than that of control mice at most time points, the weights were near or exceeded the upper limit reported for CD1 mice.5 The 21-d-old male CD1 mice in our study weighed between 13.1 and 20.3 g, and our 21-d-old female CD1 mice weighed between 12.3 and 17.8 g; the CRL website5 states that the reference weight (that is, mean ± 2 SD) of 21-d-old CD1 mice is 9 to17 g. Therefore, we conclude the difference in the weight of the group B mice was clinically irrelevant and should not affect the age at which MuAstV-infected CD1 mice are weaned. However, MuAstV-induced decreased weight gain might have a clinically relevant effect for mouse genotypes already prone to low pup weight gain and poor weaning performance, especially in male pups. Our data found that the most significant factor in determining differences in pup weight in both infected and uninfected female and male pups was the day on which the litter was born during the 3-d window. Although the weights of mice did not differ significantly during the first week of life, the birthdate-associated difference became significant starting at PND 11 and continuing through PND 28 in female mice and through PND 42 in male mice. The prolonged gestation of the mice born on the third day appeared to predispose them to greater weight gain. Therefore, when designing an experiment, it is important to carefully assign mice to control and experimental groups to limit as many factors as possible. Although we would not have predicted that day of birth would affect weight gain, this factor distributed equally across the groups and did not influence the data analysis of the mice infected at PND 3 and 13 because mice born on each day were assigned to both control and experimental groups.
Here we report the first histopathologic analysis of MuAstV-infected mice. No abnormal histopathologic changes or differences beyond age-associated lesions were observed in any of the intestines, liver, spleen, or kidney samples examined from control or MuAstV-infected mouse pups at 4 to 12 DPI regardless of whether they were infected at PND 3 or 13. Our data show that MuAstV caused a subclinical infection, primarily in the intestine, with varying levels of MuAstV RNA detectable in the intestines of all pups and trace levels of MuAstV RNA detectable in the spleen, liver, and kidney of some pups. In mice infected at PND 3, levels of MuAstV RNA were low in the intestine at 4 and 8 DPI and MuAstV was detected in the spleen and liver of only a single mouse. By 12 DPI, the levels of MuAstV RNA had risen substantially (approximately 10,000-fold) from those on DPI 4. Our study shows that oral inoculation of mice on PND 3 with MuAstV resulted in only low-level intestinal infection, which peaked at 12 DPI (PND 15), and did not readily disseminate to other tissues. Furthermore, after PND 11, when developmental changes in the intestine have occurred that allow for the digestion of solid food and feces8 (coprophagy), MuAstV infection increased markedly. In addition, these developmental changes might make the intestinal cells targeted by MuAstV more susceptible to infection. In contrast, in mice infected at PND 13, levels of MuAstV RNA were high in the intestines of all mice at 4 and 8 DPI (PND 17 and 21), and MuAstV RNA was detected in the spleen, liver, and kidney of several mice at these time points. By 12 DPI (PND 25), the levels of MuAstV RNA in the intestine dropped substantially (approximately 100-fold) from those on 4 DPI (PND 17). Our data suggest that oral inoculation of mice on PND 13 with MuAstV resulted in robust infection in the intestines, with dissemination to other tissues, but infection was cleared from the other tissues by 12 DPI. In both groups of pups, the highest levels of MuAstV RNA occurred between PND 15 and 21. In a previous study, adult B6 mice were infected by cohousing with MuAstV-infected Rag mice,25 and MuAstV RNA was detected on 14 DPI predominantly in the duodenum, terminal ileum, proximal colon, and MLN, with lower levels of MuAstV RNA in the distal colon and spleen. In that same study,25 MuAstV had disseminated to the spleen, kidney, liver, and MLN of Rag1 mice by 14 DPI, suggesting that MuAstV persisted longer in the spleens of B6 and Rag mice infected as adults as compared with CD1 mice infected as pups.
Two previous studies monitored the time course of shedding of MuAstV in feces from adult mice. MuAstV was detected in the feces of 8- to 12-wk-old C57BL/6 and Stat1 mice exposed to MuAstV-infected Rag mice from 2 to 14 d after exposure,25 and MuAstV was detected in the feces of experimentally infected 3- to 6-wk-old C57BL/6 mice from 2 to 39 DPI.17 We obtained moderate to high levels of MuAstV RNA from the feces of all CD1 dams through PND 35 (22 and 32 DPI), and low levels of MuAstV RNA were detected in about half of the dams through the end of the study (that is, DPI 29 or 39). Peak levels of MuAstV RNA were significantly higher in the group B dams (inoculated on PND 13 of their litters) than those in group A dams (inoculated on PND 3) at 5 and 7 DPI. Although we cannot rule out the possibility that dams in the 2 groups received slightly different doses of MuAstV, given that they were inoculated on different days, it seems more likely that the presence in the group B cages of the ten 13- to 21-d-old pups with high levels of MuAstV in their intestines amplified the infection in the group B dams. Once pups were weaned, MuAstV RNA levels were equivalent between group A and group B dams. Low levels of MuAstV RNA (median, 150 U) were detected in almost all CD1 weanling mice, which were infected with MuAstV as pups, on PND 21 through 63. At PND 21, when pups were weaned, MuAstV RNA levels in the dams were approximately 200 times greater than in the weanlings, whereas at PND 28 and 35, MuAstV RNA levels in the dams were approximately 20 and 10 times greater than in the weanlings. Only on PND 42 did MuAstV RNA levels in the feces of weanlings exceed those in the dams.
Most (87%) of the 144 sentinels exposed to soiled bedding from cages housing dams with litters, dams alone, or weanlings became infected with MuAstV, confirming that MuAstV in feces, detected by RT-PCR analysis between PND 7 and 42, was infectious and present in sufficient amounts to cause infection of sentinel mice for at least 6 wk. In addition, most sentinels (75%) exposed to soiled bedding that had been aged 1, 2, or 3 wk became infected, indicating that feces present in cages at the routine cage-change interval of 14 d likely still contains infectious MuAstV. This finding is not unexpected, given that human astrovirus stored in clean groundwater at 20 °C showed no decrease in infectivity after 15 d but a 10-fold decrease after 30 d.9 Therefore, the use of soiled-bedding sentinels is an effective method for monitoring for MuAstV in a colony of laboratory mice.
Many laboratory animal programs are moving away from the use of soiled-bedding sentinels toward environmental monitoring for routine microbiologic monitoring. We found that, when using swabs and RT-PCR analysis, monitoring of cage surfaces at 7 to 8 d after addition of MuAstV-infected mice to the cage was effective, because swabs from all cages tested on PND 10 to 61 were positive for MuAstV RNA, with the exception of the swabs from the cage housing the group A dam that did not become infected. Environmental air monitoring of the IVC exhaust manifolds for MuAstV RNA by using swabs was variably effective depending on the location of the exhaust manifold swabbed. Between 21 and 56 DPI, all 6 swabs taken from the rear exhaust manifold near the junction of the exhaust hose at the top of the rack of the single-sided IVC rack were positive for MuAstV RNA, whereas fewer than half of the swabs taken from 3 locations on the exhaust manifold at the end of the same IVC rack were positive for MuAstV RNA. It is important to realize it took 3 wk for detectable levels of dust and debris to be deposited on the exhaust manifold surface; therefore during the acute phase of MuAstV infection, exhaust manifold swabs might miss infected mice on the IVC rack. This timeframe for the accumulation of detectable levels of MuAstV is longer than reported for fur mites and Corynebacterium bovis.12,14 Specifically, fur mite DNA was detected in more than half of the IVC horizontal exhaust manifold swabs after 1 wk of dust and debris accumulation from a single cage of fur mite infested mice and 69 cages containing only bedding.12 Furthermore, C. bovis was detected on the horizontal exhaust manifold of an IVC rack after 6 d of dust and debris accumulation from a single cage of infected nude mice and empty sterile cages during the early stages of C. bovis infection.14 In a recent study, mice infected or infested with 9 murine pathogens (murine norovirus, mouse parvovirus, mouse hepatitis virus, Helicobacter spp., Pasteurella pneumotropica, Entamoeba muris, Tritrichomonas muris, pinworms, and fur mites) were housed on an IVC rack from a different manufacturer than in the other cited and current studies, and analyses of swabs of the exhaust plenum were unable to detect any of the agents over a 12-wk period. This finding underscores the need to validate environmental testing in multiple IVC models.3 In addition, exhaust air monitoring using the sampling of filters in the exhaust air stream has proven to be effective for detecting murine infections.3,6,18,19,26 Exhaust air detection of MuAstV appears promising, but more extensive testing in the current and other IVC rack types by using multiple sampling sites and methods is needed to more accurately determine the usefulness of this method.
In conclusion, MuAstV causes a subclinical chronic infection, lasting at least 3 wk, primarily in the intestines of CD1 mice, regardless of the age at infection. Soiled-bedding sentinels and RT-PCR analysis of feces, cage swab material, and exhaust air debris are all effective for detecting MuAstV.
Acknowledgments
This work was funded by grants from the American College of Laboratory Animal Medicine and Grants for Laboratory Science (GLAS) from the American Association for Laboratory Animal Science. We thank Alison Faruolo for technical assistance.
References
- 1.Barthold SW. 1987. Host age and genotypic effects on enterotropic mouse hepatitis virus infection. Lab Anim Sci 37:36–40. [PubMed] [Google Scholar]
- 2.Barthold SW, Griffey SM, Percy DH. 2016. Pathology of laboratory rodents and rabbits, 4th ed Ames (IA): Wiley–Blackwell. [Google Scholar]
- 3.Bauer BA, Besch-Williford CL, Livingston RS, Crim MJ, Riley LK, Myles MH. 2016. Influence of rack design and disease prevalence on detection of rodent pathogens in exhaust air debris samples from individually ventilated caging systems. J Am Assoc Lab Anim Sci 55:782–788. [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch A, Pinto RM, Guix S. 2014. Human astroviruses. Clin Microbiol Rev 27:1048–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles River Laboratories International. [Internet]. 2017. CD1 IGS mouse. [Cited 20 January 2017] Available at: www.criver.com/files/pdfs/rms/cd1/rm_rm_d_cd1_mouse.aspx.
- 6.Compton SR, Homberger FR, Paturzo FX, Clark JM. 2004. Efficacy of 3 microbiological monitoring methods in a ventilated cage rack. Comp Med 54:382–392. [PubMed] [Google Scholar]
- 7.De Benedictis P, Schultz-Cherry S, Burnham A, Cattoli G. 2011. Astrovirus infections of humans and animals—molecular biology, genetic diversity, and interspecies transmissions. Infect Genet Evol 11:1529–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebino KY. 1993. Studies on coprophagy in experimental animals. Jikken Dobutsu 42:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Espinosa AC, Mazari-Hiriart M, Espinosa R, Maruri-Avidal L, Mendez E, Arias CF. 2008. Infectivity and genome persistence of rotavirus and astrovirus in groundwater and surface water. Water Res 42:2618–2628. [DOI] [PubMed] [Google Scholar]
- 10.Farkas T, Fey B, Keller G, Martella V, Egyed L. 2012. Molecular detection of novel astroviruses in wild and laboratory mice. Virus Genes 45:518–525. [DOI] [PubMed] [Google Scholar]
- 11.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 12.Jensen ES, Allen KP, Henderson KS, Szabo A, Thulin JD. 2013. PCR testing of a ventilated caging system to detect murine fur mites. J Am Assoc Lab Anim Sci 52:28–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Kjeldsberg E, Hem A. 1985. Detection of astroviruses in gut contents of nude and normal mice. Brief report. Arch Virol 84:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manuel CA, Pugazhenthi U, Leszczynski JL. 2016. Surveillance of a ventilated rack system for Corynebacterium bovis by sampling exhaust-air manifolds. J Am Assoc Lab Anim Sci 55:58–65. [PMC free article] [PubMed] [Google Scholar]
- 15.Maronpot RR, Boorman GA, Gaul BW. 1999. Pathology of the mouse: reference and atlas. St Louis (MO): Cache River Press. [Google Scholar]
- 16.Martin F, Keraney JF. 2002. Marginal-zone B Cells. Nat Rev Immunol 2:323–335. [DOI] [PubMed] [Google Scholar]
- 17.Marvin SA, Huerta CT, Sharp B, Frieden P, Cline TD, Schultz-Cherry S. 2015. Type I interferon response limits astrovirus replication and protects against increased barrier permeability in vitro and in vivo. J Virol 90:1988–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller M, Ritter B, Zorn J, Brielmeier M. 2016. Exhaust air dust monitoring is superior to soiled bedding sentinels for detection of Pasteurella pneumotropica in individually ventilated cage systems. J Am Assoc Lab Anim Sci 55:775–781. [PMC free article] [PubMed] [Google Scholar]
- 19.Miller M, Ritter B, Zorn J, Brielmeier M. 2016. Exhaust air particle PCR detects Helicobacter hepaticus infections at low prevalence. J Vet Sci Technol 7:4–5. [Google Scholar]
- 20.Moser LA, Carter M, Schultz-Cherry S. 2007. Astrovirus increases epithelial barrier permeability independently of viral replication. J Virol 81:11937–11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng TF, Kondov NO, Hayashimoto N, Uchida R, Cha Y, Beyer AI, Wong W, Pesavento PA, Suemizu H, Meunch MO, Delwart EL. 2013. Identification of an astrovirus commonly infecting laboratory mice in the US and Japan. PLoS One 8:e66937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan TG, Kapusinsky B, Wang C, Rose RK, Lipton HL, Delwart EL. 2011. The fecal viral flora of wild rodents. PLoS Pathog 7:e1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pillai S, Cariappa A, Moran ST. 2005. Marginal zone B cells. Annu Rev Immunol 23: 161–196. [DOI] [PubMed] [Google Scholar]
- 24.Wolf JL, Cukor G, Blacklow NR, Dambrauskas R, Trier JS. 1981. Susceptibility of mice to rotavirus infection: effects of age and administration of corticosteroids. Infect Immun 33:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoyama CC, Loh J, Zhao G, Stappenbeck TS, Wang D, Huang HV, Virgin HW, Thackray LB. 2012. Adaptive immunity restricts replication of novel murine astroviruses. J Virol 86:12262–12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorn J, Ritter B, Miller M, Kraus M, Northrup E, Brielmeier M. 2016. Murine norovirus detection in the exhaust air of IVCs is more sensitive than serological analysis of soiled bedding sentinels. Lab Anim 51:301–310. [DOI] [PubMed] [Google Scholar]