Abstract
The “Molecular Imaging in Nanotechnology and Theranostics” (MINT) Interest Group of the World Molecular Imaging Society (WMIS) was founded in 2015 and was officially inaugurated during the 2016 World Molecular Imaging Conference (WMIC). The MINT interest group was created in response to the exponential growth of the fields of Nanotechnology and Theranostics in recent years, and the resulting need to provide a more organized and focused forum on these topics at the WMIS and the WMIC. The overarching goal of MINT is to bring together the many scientists who work on molecular imaging approaches using nanotechnology, and those that work on theranostic agents. MINT therefore represents scientists, labs, and institutes that are very diverse in their scientific backgrounds and areas of expertise, reflecting the wide array of materials and approaches that drive these fields. In this short review, we attempt to provide a condensed overview over some of the key areas covered by MINT. Given the breadth of the fields and the given space constraints, we have limited the coverage to the realm of nano-constructs, although theranostics is certainly not limited to this domain. We will also focus only on the most recent developments of the last 3-5 years, in order to provide the reader with an intuition of what is “in the pipeline” and has potential for clinical translation in the near future.
Introduction
With unique properties endowed by their size, modular structure, and functionalization abilities, biomedical nanoparticles are being unremittingly developed and used in biomedicine. In medical imaging, they serve as contrast agents—detectable with multiple modalities simultaneously—and give rise to new techniques for the ever-richer acquisition of molecular information. Some are already employed clinically as therapeutics, or delivery vehicles for pharmaceuticals, since they serve to reduce systemic side effects [1]. Targeted drug- and gene-delivery strategies and stimuli-responsive nanoparticle therapies are in clinical trials [2]. Modular and versatile, unifying imaging with therapy, nanoparticles are becoming true theranostic agents.
Compared to small molecules, nanoparticles feature notable advantages as theranostic agents, summarized in Figure 1: (1) their modular structure and surface modifications enable multiple functionalities (decreased immunogenicity, targeting, multimodal imaging, therapy, and controlled pharmacokinetics); (2) specific tissues can be targeted passively (e.g. reticuloendothelial system or kidneys), as can many tumors through the ‘enhanced permeability and retention’ (EPR) effect; (3) Nanoparticles can respond to their microenvironment or to external stimuli to provide therapy and contrast only where needed [3]; and (4) different types of therapy can be elicited by the Nanoparticles. These features render Nanoparticles as peerless imaging agents using traditional medical imaging, and enable the development of new modalities and theranostic applications. Many strategies have been reported for creating biomedical imaging nanoparticles using a variety of materials, and this has generated a virtual cornucopia of easily obtainable nanoparticle agents, schematically depicted in Figure 2. A non-exhaustive selection of references, tabulated by material, imaging modality, and therapy is presented in Table 1.
Fig. 1.
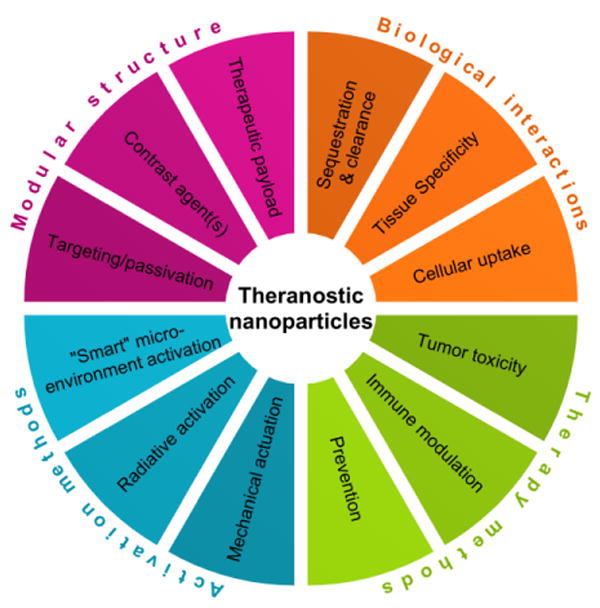
Through their modular structure, nanoparticles can incur specific biological interactions and deliver targeted therapy using intrinsic markers or external stimuli.
Fig. 2.

Nanoparticles of different materials, with many applications, are available at our fingertips.
Table 1.
Biomedical nanoparticles developed from a wide variety of materials recently reported for theranostic applications. Nanoparticles with multiple functionalities are listed multiple times.
| Noble metals | Other metals | Metal oxides | Semiconductors | Silica | Carbon | Polymers / Liposomes | Proteins | Other materials | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Imaging | MRI | [40, 55, 157] | [26, 28, 40, 88, 133, 158-161] | [6-12, 26, 28, 40, 118, 121, 123, 133, 158-160, 162-168] | [39-40] | [55] | [40] | [126, 165] | [82, 169-170] | |
| PET/SPECT | [16-17, 25, 29-30, 40, 101-102, 142, 171] | [24, 26, 28, 40, 158-159, 172-174] | [24, 26, 28, 40, 158-159, 162, 172-175] | [18, 40] | [20, 27, 34-35] | [40, 109, 136] | [21, 23, 99, 102, 106, 134-135] | [32, 176] | [22, 33, 131-132, 135, 145, 169] | |
| CT | [17, 30, 62, 65, 137-139, 142, 171] | [80, 118-120] | [18] | [20, 27, 35] | [62, 80, 138] | [135, 143, 145] | ||||
| Optical | [36, 49-50, 52, 55-60, 146, 177] | [36, 108, 117] | [36, 78, 116] | [19, 39] | [34-35, 49-50, 52, 55-60, 156, 177] | [109] | [38, 43, 78, 105, 107, 126-127, 129, 146, 178] | [116, 176] | [145, 169] | |
| Photo-acoustic | [62-67] | [79, 88] | [78, 80, 90-91, 93] | [72-73, 83-85] | [92] | [87, 151] | [43, 62, 67-69, 71-73, 75, 78-80, 83-85, 93, 106] | [76] | [74, 77, 81-82, 86, 145] | |
| Ultrasound | [78, 165] | [78, 126-127, 165] | ||||||||
| Cerenkov | [30, 101-102, 104, 171] | [24] | [24, 98, 103, 162, 175] | [18-19] | [100] | [99, 102, 135] | [98] | [145, 179-180] | ||
| Therapy | Drug delivery | [66] | [166] | [92] | [136] | [105-106, 125, 127] | ||||
| Gene/siRNA delivery | [146] | [108] | [109] | [99, 105, 107, 146, 178] | ||||||
| Immuno-therapy | [101] | [12] | [130] | [129-130] | [181] | |||||
| PTT/PDT | [16, 63, 65, 67, 110-113, 146, 157] | [88, 117, 174] | [78, 80, 98, 116, 174] | [73, 83-84] | [100] | [67, 73, 75, 78, 80, 83-84, 129, 146] | [76, 98, 116] | [82, 86, 143] | ||
| Radio-therapy | [137-142] | [133] | [116, 118, 133] | [135, 138] | [116] | [143] | ||||
| Externally actuated | [114-115] | [118-121, 123] |
Imaging
Among the first nanoparticle structures to allow molecular imaging were superparamagnetic iron oxide nanoparticles (SPIONs), used for contrast generation with magnetic resonance imaging (MRI) [4-5]. The current emphasis lies on their clinical translation, especially given the renaissance spurred by Ferumoxytol, which is now FDA-approved for systemic injection as an iron replacement therapy (trade name Feraheme). As a member of the family of ultrasmall superparamagnetic iron oxide nanoparticles (USPIOs), ferumoxytol causes regional T1 and T2* shortening in vivo, leading to signal enhancement or loss on conventional MR pulse sequences [6]. Ferumoxytol has shown promise in diverse areas such as noninvasive identification of Type 1 Diabetes [7], determining the severity of neurological diseases [8], imaging of tumor-associated macrophages (TAMs) [9], cell tracking [10], or whole-body cancer staging [11]. A recent and very exciting discovery was the finding that ferumoxytol may have an intrinsic, anti-cancer therapeutic effect [12]: intravenous ferumoxytol administration was shown to prevent metastases to the liver. This phenomenon is thought to be due to pro-inflammatory macrophage (M1) polarization in tumor tissues [12]. Such discoveries bear the hope that other nanoparticle agents may also harbor such unexpected theranostic effects.
Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are noninvasive imaging modalities frequently used in clinical settings for oncology, neuroimaging, cardiology, etc. [13-14] using radioactive nuclides conjugated to small molecules (e.g. 18F-fluorodeoxyglucose (18F-FDG)) or antibodies. Recently, nanoparticles labeled with radiotracers were found to be very promising – particularly in cancer imaging – in preclinical studies because of three major advantages: (1) the EPR effect; (2) high surface-to-volume ratio of the nanoparticles allowing high density radiolabeling either using chelators (such as DOTA), chelator-free strategies, or intrinsic labeling during synthesis [15]; and (3) complementary multimodal imaging. Many different radionuclide-nanoparticle combinations have been reported; but smaller, rapidly clearable nanoparticles with fast decaying radiolabels are ideal for potential clinical translation. Nanoparticles with 64Cu (t1/2 = 12.7 hours) were shown to allow in vivo imaging up to 48 hours [16-21]. Ultra-small chelator-free renally clearable 64Cu-labeled Cu nanoclusters were reported for imaging in orthotopic lung cancer mouse models [22]. In another study, 64Cu based liposomes were used to assess the EPR effect in canine cancer models, suggesting high intertumoral heterogeneity of EPR-based uptake [23]. Other radioisotope-nanoparticle combinations have been employed for different imaging purposes, including 68Ga (t1/2 = 68 minutes), 89Zr (t1/2 = 3.3 days), 111In (t1/2 = 2.8 days), 198Au (t1/2 = 2.69 days) [24-30]. Recently, 89Zr was used to label soft, polymeric and lipoprotein nanoparticles for imaging of TAMs [31-33]. Important steps towards clinical translation of PET nanoparticles are being carried out using “Cornell dots” (C-dots) labeled with 124I (t1/2 = 4.18 days), showing excellent localization and no toxicity in metastatic melanoma patients [34-35].
Fluorescent nanoparticles (FNPs), with intrinsic fluorescence or loaded with fluorescent dyes, are being investigated as imaging agents due to their advantages over small molecule dyes, namely: improved specificity (by active or passive targeting [36-37]), increased circulation time (by evading immune detection and renal clearance), and smart activation (by pH dependency or enzymatic activity) as well as increased signal intensity [36, 38]. FNPs have been reportedly used for sentinel lymph node (SLN) and solid tumor detection, image-guided tumor surgery with real-time feedback, and monitoring of drug delivery [36, 39]. Also fluorescent, quantum dots (q-dots) and gold nanoparticles (passivated with polyethylene glycol (PEG) or silica) [36, 39] can be combined with complementary imaging agents like gadolinium [39-40], organic dyes [39], polymeric p-dots [39, 41], or fluorescent proteins [39, 42-43]. Q-dots are of particular interest due to their broad absorption and size-tunable emission spectra, making them suitable for multiplexing [37, 44] and less susceptible to photobleaching compared to broadly used organic dyes [37].
A new generation of fluorogens has been introduced to bioimaging with FNPs, exhibiting aggregation-induced emission (AIE) [45]. AIE overcomes aggregation-induced quenching and allows for higher concentration of fluorogens on the NP surface while also reducing photobleaching [45]. However, due to the fluorogens' broad emission spectra, it is less suitable for multiplexing, instigating some groups to work on narrowing the emission spectra via Förster resonance energy transfer [46]. Although fluorescence imaging is complicated by high false-positive rates [47], improvements in specificity have been reported, leading to higher levels of complete tumor resection [48].
Raman imaging – a spectroscopic optical imaging technique – with surface enhanced (resonance) Raman scattering nanoparticles (SE(R)RS NPs) shows much potential for in vivo imaging of cancer [49]. Unlike fluorescent agents, SERS NPs do not suffer from significant background from endogenous molecules or photobleaching [50]. In fact, the endogenous Raman signals can be used by the detection hardware to generate surface topology on which the specific signals can be mapped without interference [51]. With their low detection threshold and high signal specificity SERRS NPs were shown to delineate tumors – and even premalignant lesions – passively through the EPR effect [52]. Nanoparticle sequestration by the RES allows for imaging of SLNs [53] and tumors in the liver and spleen [54]. When combined with active targeting via antibodies, peptides, or aptamers SERRS NPs were reported to delineate tumors preoperatively and intraoperatively [55], as well as detecting microscopic tumors and metastatic foci in glioblastoma, ovarian cancer, and lung metastases [56-59, 60 ]. Given their potential significance in tumor imaging and non-toxic composition, the timely translation of SERS NPs into the clinic could represent a fundamental improvement in patient morbidity and mortality.
Nanoparticles can be engineered to allow “hybrid” imaging methods, where excitation and detection occur through distinct physical processes. For example, in photoacoustic imaging (PAI), pulses of light excite the contrast agent, which in turn produces a mechanical response, detected as ultrasound [61]. Images are obtained noninvasively, deeper within tissues and with higher spatial resolution compared to purely optical imaging techniques. Nanoparticles based on plasmonic [62-67], polymeric [68-73], and other materials [74-88] have all shown great potential in preclinical studies, combining photoacoustic detection with photothermal and photodynamic therapies (PTT and PDT). However, nanoparticles based on iron oxide [89-91] or silica [92] have a higher likelihood for clinical translation as similar materials are already approved for use in humans. Magnetically actuated photoacoustically-active nanoparticles are of particular interest, as they allow more specific detection through magnetic actuation [93].
Some charged particles produced by radionuclide decay emit visible light, referred to as Cerenkov luminescence (CL) [94], already demonstrated for cancer imaging in humans using 18F-FDG [95]. Nanoparticle-based agents bring great new potential to this emerging modality, allowing for more specific imaging [96-98] and therapy [99-101], while active or passive targeting [102] can map receptors [103] or enzymes [104] of interest. As nanoparticles for PET imaging are translated to the clinic, CL will undoubtedly follow suit, providing additional, complementary information to the benefit of the patient.
Therapy
Nanoparticles have great potential as therapeutic agents, delivering drugs, genes, or other forms of therapy, with many examples of clinical success [1]. In the paradigm set by Doxil, nanoparticles can be engineered to encapsulate pharmaceuticals (such as doxorubicin (DOX)) and release them at targeted sites, reducing systemic toxicity and improving pharmacokinetic profiles. In a newer scheme, DOX-conjugated poly(lactic-co-glycolic acid) (PLGA) was loaded into an injectable nanoparticle generator spontaneously releasing nanoparticles upon pH stimulation, which are later cleaved into DOX within the cell to avoid drug efflux pumps, showing enhanced efficacy in metastatic breast cancer models over free DOX [105]. Ultrasmall 64Cu-PEG-melanin nanoparticles loaded with FDA-approved multikinase inhibitor sorafenib provide PET-PAI image-guided chemotherapy in liver xenograft models [106]. Recently, siRNA-loaded nanoparticles were employed in various settings, such as gene delivery into lung cancer cells – but not normal cells – without targeting ligands [107]; transdermal application for suppressing EGFR expression and downstream ERK signaling in mice and humans with no clinical or histological toxicity [108]; and increasing progression-free survival in murine acute kidney injury models and nonhuman primates [109].
Photothermal therapy (PTT) employs heat to destroy cancer cells and nanomedicine can substantially facilitate this process. AuroShell is the first-demonstrated exogenous vis-NIR light absorbing nanoparticle for photothermal tumor ablation and optical coherence tomography (OCT) [110] and was extensively investigated in murine and canine models of various cancer types [111-113]. Pilot clinical studies are being conducted in head and neck cancer (NCT00848042), lung cancer (NCT01679470) and prostate cancer (NCT02680535). Gold nanoparticles with radiofrequency waves can also induce PTT [114-115]. For photodynamic (PDT) therapy, Cerenkov luminescence can activate transferrin-coated TiO2 photosensitizer nanoparticles and mediate tumor remission by generating free radicals and immune cell infiltration [116]. NIR PDT is achievable using photosensitizing silica-coated upconversion nanoparticles for deeper tissue penetration than visible light [117]. Aminosilane-coated iron oxide (NanoTherm), significantly polarized under external magnetic fields to selectively ablate tumors by heat generation, is undergoing clinical trials in the USA [118-120]. FDA-approved SPIONs were also found to inhibit tumor growth by hyperthermia under magnetic fields at preclinical levels [121-123]. The integration of multiple energies and modalities is a unique characteristic of nanoparticles that will make them invaluable for detection and therapy in a wide variety of diseases.
Ultrasound (US) can promote nanoparticle accumulation and drug release in tumors through cavitation and is reported to mediate nanoparticles crossing even intact blood-brain barriers [124] — see minireview on US molecular imaging by Caskey in this issue. Nanoparticles as a sonoporation enhancer are advantageous over microbubbles (MBs) for their extravasation in capillaries and sustained activity. PEG-PDLA nanoparticles were used to overcome aqueous solubility barriers of paclitaxel under US guidance [125]. Polymer nanoparticle-stabilized MBs with embedded SPIONs provide MRI/US imaging and pulse-activated nanoparticle release [126]. Gas-generating docetaxel (DTX) and Cy5.5 dye-loaded poly(CBL-PO) nanoparticles in MBs offer fluorescence/US signal and are released at tumor sites upon ultrasound irradiation through bubble burst, with much higher contrast than clinically-used Sonovue® and Definity® and higher therapeutic effects than free DTX or without US activation [127].
Besides the aforementioned hyperthermia effects, SPIONs were recently found to intrinsically inhibit tumor growth as a potential macrophage-modulating immunotherapy [12]. Adjuvant drug labeled liposome- and lipid-based nanoparticles covalently attached to cell surface for adoptive T-cell therapy can markedly decrease tumor burden at preclinical setups [128]. PEG-PLGA nanoparticles encapsulated with indocyanine green (ICG) and TLR7 agonist R837 can generate tumor-associated antigens during PTT and its combination therapy with anti-CTLA4 antibodies can significantly inhibit metastasis in a 4T1 orthotopic model [129]. Carbon nanotube-PLGA nanocomplexes with high surface area were functionalized with T-cell stimulating antigens for delivery of IL-2 at a dose much lower than clinically used to overcome adverse reactions. These nanocomplexes generate a large number of cytotoxic T-cells and delay tumor growth in murine melanoma models [130].
PET-based nanoparticles are being perused as an alternative to traditional internal radiotherapy or brachytherapy as they allow even distribution within the tumor volume. Alpha emitters with long half-life like 225Ac (t1/2 = 10 days) are the preferred radionuclides for the formulation of nanoparticles [131-132]. However, there is concern about the radioactivity of downstream decay processes. Recently, 131I (t1/2 = 8 days), often used for the treatment of thyroid cancer, has been integrated with iron oxide nanoparticles and polymeric nanoparticles for targeted therapy of hepatocellular carcinoma in mouse models [133-134]. 177Lu (t1/2 = 6.6 days) has been utilized in lipid-calcium phosphate nanoparticles showing significant growth inhibition in subcutaneous xenograft tumor models [135]. There is also great deal of interest in using a nonradioactive module as a therapeutic partner in the formulation of PET nanoparticles. In a recent study, a 99mTc-labeled (t1/2 = 6 hours), folic acid targeted, multiwalled CNT nanoprobe has been developed using Methotrexate as a therapeutic module showing an augmentation of the therapeutic efficacy of the drug in the presence of 99mTc [136].
Metal nanoparticles can act as radiosensitizers, enhancing the efficacy of radiotherapy, e.g. renally clearable ultrasmall gold nanoclusters with high tumor uptake [137], or polymeric nanoparticles loaded with gold nanoparticles [138]. Gold nanoparticles in glioma models boosted overall survival in mice [139], as well as in head and neck cancer models [140], while silver nanoparticles produced similar results in glioma-bearing rats [141]. Therapeutic 103Pd-Au nanoseeds offer SPECT signal along with a radiotherapeutic effect of >80% tumor shrinkage [142]. 131I-doped CuS nanoparticles provide combined PTT and radiotherapy together with CT and gamma image-guidance to treat 4T1 subcutaneous and metastatic tumors [143].
Future Directions
Nanoparticles are quickly becoming universal imaging agents, taking multimodal imaging to new heights [144-145]. Multiple treatments can be packaged within the same nanoparticle agent, for example, a prophylactic hydrogel patch containing fluorescently-labeled targeted gold nanoparticles locally implanted into a tumor makes a triple combination therapy of siRNA against Kras, VEGF inhibitor delivery, and PTT available at the same time for colon cancer treatments [146]. Monitoring the nanoparticle distribution in the body, but also the therapeutic load delivery via imaging is possible, for example via MRI [147-148]. The next crucial step will be predicting their distribution even before administration, especially given the high variability of the EPR effect. Such prediction may take the form of “companion-nanoparticles”[149] or computational models [150]. Bioderived nanoparticles, synthesized using “green chemistry” or cells [151-153] can expand the limits of biocompatibility and immune evasion through biomimicry [154]. Such biocompatible synthesis approaches could soon enable chemical manipulation of nanoparticles in vivo [155]. As nanoparticles transition to the clinic, they are investigated more deeply and new effects are discovered. For example, besides their role in intraoperative image-guided oncosurgeries, C-dots were recently observed to induce ferroptosis of cancer cells both in vitro and in vivo [156], and iron oxide nanoparticles were shown to reprogram TAMs to attack tumors [12].
Conclusion
With many examples of successful nanoparticle theranostic agents already employed clinically, several undergoing clinical trials, and countless others emerging from preclinical studies, we are ushering in the era of nanomedicine. Smart, specific, and customizable, theranostic nanoparticles will soon—pending FDA approval—detect, treat, and prevent disease. MINT has been established to create a forum to discuss these advances and better integrate disciplines that develop and use nanoparticles for imaging and therapy.
Acknowledgments
The following funding sources (to M.F.K.) are acknowledged: NIH R01 EB017748 and K08 CA16396; M.F.K. is a Damon Runyon-Rachleff Innovator supported (in part) by the Damon Runyon Cancer Research Foundation (DRR-29-14); Pershing Square Sohn Prize by the Pershing Square Sohn Cancer Research Alliance; MSKCC Center for Molecular Imaging & Nanotechnology (CMINT) Grant; MSKCC Technology Development Grant; Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and The Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center. Acknowledgements are also extended to the grant-funding support provided by the MSKCC NIH Core Grant (P30-CA008748).
Footnotes
Conflicts of Interest: M.F.K. is an inventor on several pending patents related to Raman nanoparticles, Raman detection and theranostic hardware, as well as radiolabeling of silica particles, and is a co-founder of RIO Imaging, Inc., a startup company that has licensed several of these patents.
References
- 1.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res. 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 2.Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioengineering & Translational Medicine. 2016;1:10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stylianopoulos T. Intelligent drug delivery systems for the treatment of solid tumors. Eur J Nanomed. 2016;8:9–16. [Google Scholar]
- 4.Kircher MF, Willmann JK. Molecular body imaging: MR imaging, CT, and US. Part II. Applications. Radiology. 2012;264:349–368. doi: 10.1148/radiol.12111703. [DOI] [PubMed] [Google Scholar]
- 5.Kircher MF, Willmann JK. Molecular body imaging: MR imaging, CT, and US. part I. principles. Radiology. 2012;263:633–643. doi: 10.1148/radiol.12102394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashir MR, Bhatti L, Marin D, Nelson RC. Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging. 2015;41:884–898. doi: 10.1002/jmri.24691. [DOI] [PubMed] [Google Scholar]
- 7.Gaglia JL, Harisinghani M, Aganj I, et al. Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proc Natl Acad Sci U S A. 2015;112:2139–2144. doi: 10.1073/pnas.1424993112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirschbaum K, Sonner JK, Zeller MW, et al. In vivo nanoparticle imaging of innate immune cells can serve as a marker of disease severity in a model of multiple sclerosis. Proc Natl Acad Sci U S A. 2016;113:13227–13232. doi: 10.1073/pnas.1609397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng L, Stafford JH, Liu SC, et al. SDF-1 Blockade Enhances Anti-VEGF Therapy of Glioblastoma and Can Be Monitored by MRI. Neoplasia. 2016;19:1–7. doi: 10.1016/j.neo.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant LH, Jr, Kim SJ, Hobson M, et al. Physicochemical characterization of ferumoxytol, heparin and protamine nanocomplexes for improved magnetic labeling of stem cells. Nanomedicine. 2016 doi: 10.1016/j.nano.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klenk C, Gawande R, Uslu L, et al. Ionising radiation-free whole-body MRI versus (18)F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study. Lancet Oncol. 2014;15:275–285. doi: 10.1016/S1470-2045(14)70021-X. [DOI] [PubMed] [Google Scholar]
- 12.Zanganeh S, Hutter G, Spitler R, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nano. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratt EC, Shaffer TM, Grimm J. Nanoparticles and radiotracers: advances toward radionanomedicine. Wiley interdisciplinary reviews Nanomedicine and nanobiotechnology. 2016;8:872–890. doi: 10.1002/wnan.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DS, Im HJ, Lee YS. Radionanomedicine: Widened perspectives of molecular theragnosis. Nanomedicine: Nanotechnology, Biology and Medicine. 11:795–810. doi: 10.1016/j.nano.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Goel S, Chen F, Ehlerding EB, Cai W. Intrinsically radiolabeled nanoparticles: an emerging paradigm. Small. 2014;10:3825–3830. doi: 10.1002/smll.201401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X, Huang X, Yan X, et al. Chelator-Free 64Cu-Integrated Gold Nanomaterials for Positron Emission Tomography Imaging Guided Photothermal Cancer Therapy. ACS Nano. 2014;8:8438–8446. doi: 10.1021/nn502950t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Sultan D, Detering L, et al. Copper-64-alloyed gold nanoparticles for cancer imaging: improved radiolabel stability and diagnostic accuracy. Angewandte Chemie (International ed in English) 2014;53:156–159. doi: 10.1002/anie.201308494. [DOI] [PubMed] [Google Scholar]
- 18.Guo W, Sun X, Jacobson O, et al. Intrinsically Radioactive [64Cu]CuInS/ZnS Quantum Dots for PET and Optical Imaging: Improved Radiochemical Stability and Controllable Cerenkov Luminescence. ACS Nano. 2015;9:488–495. doi: 10.1021/nn505660r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Huang X, Guo J, et al. Self-Illuminating 64Cu-Doped CdSe/ZnS Nanocrystals for in Vivo Tumor Imaging. Journal of the American Chemical Society. 2014;136:1706–1709. doi: 10.1021/ja410438n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaffer TM, Harmsen S, Khwaja E, Kircher MF, Drain CM, Grimm J. Stable Radiolabeling of Sulfur-Functionalized Silica Nanoparticles with Copper-64. Nano Letters. 2016;16:5601–5604. doi: 10.1021/acs.nanolett.6b02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pressly ED, Pierce RA, Connal LA, Hawker CJ, Liu Y. Nanoparticle PET/CT Imaging of Natriuretic Peptide Clearance Receptor in Prostate Cancer. Bioconjugate Chemistry. 2013;24:196–204. doi: 10.1021/bc300473x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao F, Cai P, Yang W, et al. Ultrasmall [(64)Cu]Cu nanoclusters for targeting orthotopic lung tumors using accurate positron emission tomography imaging. ACS Nano. 2015;9:4976–4986. doi: 10.1021/nn507130k. [DOI] [PubMed] [Google Scholar]
- 23.Hansen AE, Petersen AL, Henriksen JR, et al. Positron Emission Tomography Based Elucidation of the Enhanced Permeability and Retention Effect in Dogs with Cancer Using Copper-64 Liposomes. ACS Nano. 2015;9:6985–6995. doi: 10.1021/acsnano.5b01324. [DOI] [PubMed] [Google Scholar]
- 24.Madru R, Tran TA, Axelsson J, et al. (68)Ga-labeled superparamagnetic iron oxide nanoparticles (SPIONs) for multi-modality PET/MR/Cherenkov luminescence imaging of sentinel lymph nodes. American Journal of Nuclear Medicine and Molecular Imaging. 2014;4:60–69. [PMC free article] [PubMed] [Google Scholar]
- 25.Frigell J, Garcia I, Gomez-Vallejo V, Llop J, Penades S. 68Ga-labeled gold glyconanoparticles for exploring blood-brain barrier permeability: preparation, biodistribution studies, and improved brain uptake via neuropeptide conjugation. J Am Chem Soc. 2014;136:449–457. doi: 10.1021/ja411096m. [DOI] [PubMed] [Google Scholar]
- 26.Thorek DL, Ulmert D, Diop NF, et al. Non-invasive mapping of deep-tissue lymph nodes in live animals using a multimodal PET/MRI nanoparticle. Nat Commun. 2014;5:3097. doi: 10.1038/ncomms4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaffer TM, Wall MA, Harmsen S, et al. Silica Nanoparticles as Substrates for Chelator-free Labeling of Oxophilic Radioisotopes. Nano Letters. 2015;15:864–868. doi: 10.1021/nl503522y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng J, Jia B, Qiao R, et al. In situ 111In-doping for achieving biocompatible and non-leachable 111In-labeled Fe3O4 nanoparticles. Chemical Communications. 2014;50:2170–2172. doi: 10.1039/c3cc48948e. [DOI] [PubMed] [Google Scholar]
- 29.Shukla R, Chanda N, Zambre A, et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proceedings of the National Academy of Sciences. 2012;109:12426–12431. doi: 10.1073/pnas.1121174109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black KCL, Wang Y, Luehmann HP, et al. Radioactive 198Au-Doped Nanostructures with Different Shapes for In Vivo Analyses of Their Biodistribution, Tumor Uptake, and Intratumoral Distribution. ACS Nano. 2014;8:4385–4394. doi: 10.1021/nn406258m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keliher EJ, Yoo J, Nahrendorf M, et al. 89Zr-Labeled Dextran Nanoparticles Allow in Vivo Macrophage Imaging. Bioconjugate Chemistry. 2011;22:2383–2389. doi: 10.1021/bc200405d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Medina C, Tang J, Abdel-Atti D, et al. PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. J Nucl Med. 2015;56:1272–1277. doi: 10.2967/jnumed.115.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keliher EJ, Yoo J, Nahrendorf M, et al. (89)Zr Labeled Dextran Nanoparticles Enable in vivo Macrophage Imaging. Bioconjugate chemistry. 2011;22:2383–2389. doi: 10.1021/bc200405d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benezra M, Penate-Medina O, Zanzonico PB, et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. The Journal of Clinical Investigation. 121:2768–2780. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips E, Penate-Medina O, Zanzonico PB, et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Science Translational Medicine. 2014;6:260ra149–260ra149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill TK, Mohs AM. Image-guided tumor surgery: will there be a role for fluorescent nanoparticles? Wiley interdisciplinary reviews Nanomedicine and nanobiotechnology. 2016;8:498–511. doi: 10.1002/wnan.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamila S, McEwan C, Costley D, et al. Diagnostic and Therapeutic Applications of Quantum Dots in Nanomedicine. Top Curr Chem. 2016;370:203–224. doi: 10.1007/978-3-319-22942-3_7. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Zhou K, Huang G, et al. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat Mater. 2014;13:204–212. doi: 10.1038/nmat3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Priem B, Tian C, Tang J, Zhao Y, Mulder WJ. Fluorescent nanoparticles for the accurate detection of drug delivery. Expert Opin Drug Deliv. 2015;12:1881–1894. doi: 10.1517/17425247.2015.1074567. [DOI] [PubMed] [Google Scholar]
- 40.Nune SK, Gunda P, Thallapally PK, Lin YY, Forrest ML, Berkland CJ. Nanoparticles for biomedical imaging. Expert Opin Drug Deliv. 2009;6:1175–1194. doi: 10.1517/17425240903229031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu C, Bull B, Szymanski C, Christensen K, McNeill J. Multicolor conjugated polymer dots for biological fluorescence imaging. ACS Nano. 2008;2:2415–2423. doi: 10.1021/nn800590n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sample V, Newman RH, Zhang J. The structure and function of fluorescent proteins. Chem Soc Rev. 2009;38:2852–2864. doi: 10.1039/b913033k. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Zhang Y, Gautam S, et al. Development of aliphatic biodegradable photoluminescent polymers. Proc Natl Acad Sci U S A. 2009;106:10086–10091. doi: 10.1073/pnas.0900004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Ye M, Chao Q, Jia N, Ge Y, Shen H. Simultaneous detection of multifood-borne pathogenic bacteria based on functionalized quantum dots coupled with immunomagnetic separation in food samples. J Agric Food Chem. 2009;57:517–524. doi: 10.1021/jf802817y. [DOI] [PubMed] [Google Scholar]
- 45.Yan L, Zhang Y, Xu B, Tian W. Fluorescent nanoparticles based on AIE fluorogens for bioimaging. Nanoscale. 2016;8:2471–2487. doi: 10.1039/c5nr05051k. [DOI] [PubMed] [Google Scholar]
- 46.Geng J, Zhu Z, Qin W, et al. Near-infrared fluorescence amplified organic nanoparticles with aggregation-induced emission characteristics for in vivo imaging. Nanoscale. 2014;6:939–945. doi: 10.1039/c3nr04243j. [DOI] [PubMed] [Google Scholar]
- 47.Tummers QR, Hoogstins CE, Peters AA, et al. The Value of Intraoperative Near-Infrared Fluorescence Imaging Based on Enhanced Permeability and Retention of Indocyanine Green: Feasibility and False-Positives in Ovarian Cancer. PLoS One. 2015;10:e0129766. doi: 10.1371/journal.pone.0129766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 49.Andreou C, Kishore SA, Kircher MF. Surface-Enhanced Raman Spectroscopy: A New Modality for Cancer Imaging. J Nucl Med. 2015;56:1295–1299. doi: 10.2967/jnumed.115.158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andreou C, Neuschmelting V, Tschaharganeh DF, et al. Imaging of Liver Tumors Using Surface-Enhanced Raman Scattering Nanoparticles. ACS Nano. 2016;10:5015–5026. doi: 10.1021/acsnano.5b07200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garai E, Sensarn S, Zavaleta CL, et al. High-sensitivity, real-time, ratiometric imaging of surface-enhanced Raman scattering nanoparticles with a clinically translatable Raman endoscope device. J Biomed Opt. 2013;18:096008. doi: 10.1117/1.JBO.18.9.096008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harmsen S, Huang R, Wall MA, et al. Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging. Sci Transl Med. 2015;7:271ra277. doi: 10.1126/scitranslmed.3010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spaliviero M, Harmsen S, Huang R, et al. Detection of Lymph Node Metastases with SERRS Nanoparticles. Mol Imaging Biol. 2016;18:677–685. doi: 10.1007/s11307-016-0932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreou C, Neuschmelting V, Tschaharganeh DF, et al. Imaging of Liver Tumors Using Surface-Enhanced Raman Scattering Nanoparticles. ACS Nano. 2016;10:5015–5026. doi: 10.1021/acsnano.5b07200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kircher MF, de la Zerda A, Jokerst JV, et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med. 2012;18:829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harmsen S, Bedics MA, Wall MA, Huang R, Detty MR, Kircher MF. Rational design of a chalcogenopyrylium-based surface-enhanced resonance Raman scattering nanoprobe with attomolar sensitivity. Nat Commun. 2015;6:6570. doi: 10.1038/ncomms7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang R, Harmsen S, Samii JM, et al. High Precision Imaging of Microscopic Spread of Glioblastoma with a Targeted Ultrasensitive SERRS Molecular Imaging Probe. Theranostics. 2016;6:1075–1084. doi: 10.7150/thno.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karabeber H, Huang R, Iacono P, et al. Guiding brain tumor resection using surface-enhanced Raman scattering nanoparticles and a hand-held Raman scanner. ACS Nano. 2014;8:9755–9766. doi: 10.1021/nn503948b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oseledchyk A, Andreou C, Wall MA, Kircher MF. Folate-Targeted Surface-Enhanced Resonance Raman Scattering Nanoprobe Ratiometry for Detection of Microscopic Ovarian Cancer. ACS Nano. 2017 doi: 10.1021/acsnano.6b06796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nayak TR, Andreou C, Oseledchyk A, et al. Tissue factor-specific ultra-bright SERRS nanostars for Raman detection of pulmonary micrometastases. Nanoscale. 2016 doi: 10.1039/c6nr08217c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemaster JE, Jokerst JV. What is new in nanoparticle-based photoacoustic imaging? Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2016 doi: 10.1002/wnan.1404. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheheltani R, Ezzibdeh RM, Chhour P, et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials. 2016;102:87–97. doi: 10.1016/j.biomaterials.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng X, Sun R, Yin L, Chai Z, Shi H, Gao M. Light-Triggered Assembly of Gold Nanoparticles for Photothermal Therapy and Photoacoustic Imaging of Tumors In Vivo. Advanced materials (Deerfield Beach, Fla) 2016 doi: 10.1002/adma.201604894. [DOI] [PubMed] [Google Scholar]
- 64.Dixon AJ, Hu S, Klibanov AL, Hossack JA. Oscillatory Dynamics and In Vivo Photoacoustic Imaging Performance of Plasmonic Nanoparticle-Coated Microbubbles. Small. 2015;11:3066–3077. doi: 10.1002/smll.201403398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jing L, Liang X, Deng Z, et al. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials. 2014;35:5814–5821. doi: 10.1016/j.biomaterials.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, He J, Yang K, et al. Folding Up of Gold Nanoparticle Strings into Plasmonic Vesicles for Enhanced Photoacoustic Imaging. Angewandte Chemie. 2015;127:16035–16038. doi: 10.1002/anie.201508616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang HW, Liu HL, Li ML, et al. Magnetic gold-nanorod/ PNIPAAmMA nanoparticles for dual magnetic resonance and photoacoustic imaging and targeted photothermal therapy. Biomaterials. 2013;34:5651–5660. doi: 10.1016/j.biomaterials.2013.03.085. [DOI] [PubMed] [Google Scholar]
- 68.Egusquiaguirre SP, Beziere N, Pedraz JL, Hernández RM, Ntziachristos V, Igartua M. Optoacoustic imaging enabled biodistribution study of cationic polymeric biodegradable nanoparticles. Contrast Media & Molecular Imaging. 2015;10:421–427. doi: 10.1002/cmmi.1644. [DOI] [PubMed] [Google Scholar]
- 69.Jokerst JV, Van de Sompel D, Bohndiek SE, Gambhir SS. Cellulose nanoparticles are a biodegradable photoacoustic contrast agent for use in living mice. Photoacoustics. 2014;2:119–127. doi: 10.1016/j.pacs.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li K, Liu B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chemical Society Reviews. 2014;43:6570–6597. doi: 10.1039/c4cs00014e. [DOI] [PubMed] [Google Scholar]
- 71.Lu HD, Wilson BK, Heinmiller A, Faenza B, Hejazi S, Prud'homme RK. Narrow Absorption NIR Wavelength Organic Nanoparticles Enable Multiplexed Photoacoustic Imaging. ACS Applied Materials & Interfaces. 2016;8:14379–14388. doi: 10.1021/acsami.6b03059. [DOI] [PubMed] [Google Scholar]
- 72.Xie C, Upputuri PK, Zhen X, Pramanik M, Pu K. Self-quenched semiconducting polymer nanoparticles for amplified in vivo photoacoustic imaging. Biomaterials. 2017;119:1–8. doi: 10.1016/j.biomaterials.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Yan Y, Yang Q, Wang J, et al. Heteropoly blue doped polymer nanoparticles: an efficient theranostic agent for targeted photoacoustic imaging and near-infrared photothermal therapy in vivo. Journal of Materials Chemistry B. 2016 doi: 10.1039/c6tb02652d. [DOI] [PubMed] [Google Scholar]
- 74.Maji SK, Sreejith S, Joseph J, et al. Upconversion Nanoparticles as a Contrast Agent for Photoacoustic Imaging in Live Mice. Advanced materials (Deerfield Beach, Fla) 2014;26:5633–5638. doi: 10.1002/adma.201400831. [DOI] [PubMed] [Google Scholar]
- 75.Cai X, Liu X, Liao LD, et al. Encapsulated Conjugated Oligomer Nanoparticles for Real-Time Photoacoustic Sentinel Lymph Node Imaging and Targeted Photothermal Therapy. Small. 2016;12:4873–4880. doi: 10.1002/smll.201600697. [DOI] [PubMed] [Google Scholar]
- 76.Chen Q, Liu X, Zeng J, Cheng Z, Liu Z. Albumin-NIR dye self-assembled nanoparticles for photoacoustic pH imaging and pH-responsive photothermal therapy effective for large tumors. Biomaterials. 2016;98:23–30. doi: 10.1016/j.biomaterials.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 77.Ding K, Zeng J, Jing L, et al. Aqueous synthesis of PEGylated copper sulfide nanoparticles for photoacoustic imaging of tumors. Nanoscale. 2015;7:11075–11081. doi: 10.1039/c5nr02180d. [DOI] [PubMed] [Google Scholar]
- 78.Gao S, Wang G, Qin Z, et al. Oxygen-generating hybrid nanoparticles to enhance fluorescent/photoacoustic/ultrasound imaging guided tumor photodynamic therapy. Biomaterials. 2017;112:324–335. doi: 10.1016/j.biomaterials.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 79.Ho IT, Sessler JL, Gambhir SS, Jokerst JV. Parts per billion detection of uranium with a porphyrinoid-containing nanoparticle and in vivo photoacoustic imaging. The Analyst. 2015;140:3731–3737. doi: 10.1039/c5an00207a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin Y, Li Y, Ma X, et al. Encapsulating tantalum oxide into polypyrrole nanoparticles for X-ray CT/photoacoustic bimodal imaging-guided photothermal ablation of cancer. Biomaterials. 2014;35:5795–5804. doi: 10.1016/j.biomaterials.2014.03.086. [DOI] [PubMed] [Google Scholar]
- 81.Ku G, Zhou M, Song S, Huang Q, Hazle J, Li C. Copper Sulfide Nanoparticles As a New Class of Photoacoustic Contrast Agent for Deep Tissue Imaging at 1064 nm. ACS Nano. 2012;6:7489–7496. doi: 10.1021/nn302782y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu R, Jing L, Peng D, Li Y, Tian J, Dai Z. Manganese (II) Chelate Functionalized Copper Sulfide Nanoparticles for Efficient Magnetic Resonance/Photoacoustic Dual-Modal Imaging Guided Photothermal Therapy. Theranostics. 5:1144–1153. doi: 10.7150/thno.11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lyu Y, Fang Y, Miao Q, Zhen X, Ding D, Pu K. Intraparticle Molecular Orbital Engineering of Semiconducting Polymer Nanoparticles as Amplified Theranostics for in Vivo Photoacoustic Imaging and Photothermal Therapy. ACS Nano. 2016;10:4472–4481. doi: 10.1021/acsnano.6b00168. [DOI] [PubMed] [Google Scholar]
- 84.Pu K, Mei J, Jokerst JV, et al. Diketopyrrolopyrrole-Based Semiconducting Polymer Nanoparticles for In Vivo Photoacoustic Imaging. Advanced materials (Deerfield Beach, Fla) 2015;27:5184–5190. doi: 10.1002/adma.201502285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pu K, Shuhendler AJ, Jokerst JV, et al. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nature nanotechnology. 2014;9:233–239. doi: 10.1038/nnano.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun C, Wen L, Zeng J, et al. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials. 2016;91:81–89. doi: 10.1016/j.biomaterials.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 87.Tsyboulski DA, Liopo AV, Su R, et al. Enabling in vivo measurements of nanoparticle concentrations with three-dimensional optoacoustic tomography. Journal of Biophotonics. 2014;7:581–588. doi: 10.1002/jbio.201200233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu J, Yang C, Li J, et al. Multifunctional Fe5C2 Nanoparticles: A Targeted Theranostic Platform for Magnetic Resonance Imaging and Photoacoustic Tomography-Guided Photothermal Therapy. Advanced materials (Deerfield Beach, Fla) 2014;26:4114–4120. doi: 10.1002/adma.201305811. [DOI] [PubMed] [Google Scholar]
- 89.Bogdanov AA, Jr, Dixon AJ, Gupta S, et al. Synthesis and Testing of Modular Dual-Modality Nanoparticles for Magnetic Resonance and Multispectral Photoacoustic Imaging. Bioconjugate Chemistry. 2016;27:383–390. doi: 10.1021/acs.bioconjchem.5b00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grootendorst DJ, Jose J, Fratila RM, et al. Evaluation of superparamagnetic iron oxide nanoparticles (Endorem®) as a photoacoustic contrast agent for intra-operative nodal staging. Contrast Media & Molecular Imaging. 2013;8:83–91. doi: 10.1002/cmmi.1498. [DOI] [PubMed] [Google Scholar]
- 91.Xi L, Grobmyer SR, Zhou G, Qian W, Yang L, Jiang H. Molecular photoacoustic tomography of breast cancer using receptor targeted magnetic iron oxide nanoparticles as contrast agents. Journal of Biophotonics. 2014;7:401–409. doi: 10.1002/jbio.201200155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gurka MK, Pender D, Chuong P, et al. Identification of pancreatic tumors in vivo with ligand-targeted, pH responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography. Journal of Controlled Release. 2016;231:60–67. doi: 10.1016/j.jconrel.2015.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li J, Arnal B, Wei CW, et al. Magneto-Optical Nanoparticles for Cyclic Magnetomotive Photoacoustic Imaging. ACS Nano. 2015;9:1964–1976. doi: 10.1021/nn5069258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Das S, Thorek DLJ, Grimm J. Cerenkov Imaging. Advances in cancer research. 2014;124:213–234. doi: 10.1016/B978-0-12-411638-2.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thorek DL, Riedl CC, Grimm J. Clinical Cerenkov luminescence imaging of (18)F-FDG. J Nucl Med. 2014;55:95–98. doi: 10.2967/jnumed.113.127266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Czupryna J, Kachur AV, Blankemeyer E, et al. Cerenkov-specific contrast agents for detection of pH in vivo. Journal of Nuclear Medicine. 2015;56:483–488. doi: 10.2967/jnumed.114.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grootendorst MR, Cariati M, Kothari A, Tuch DS, Purushotham A. Cerenkov luminescence imaging (CLI) for image-guided cancer surgery. Clinical and Translational Imaging. 2016;4:353–366. doi: 10.1007/s40336-016-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kotagiri N, Sudlow G, Akers W, Achilefu S. Depth-independent phototherapy using Cerenkov radiation and Titanium dioxide nanoparticles. Journal of Nuclear Medicine. 2015;56:643–643. [Google Scholar]
- 99.Black KCL, Ibricevic A, Gunsten SP, et al. In vivo fate tracking of degradable nanoparticles for lung gene transfer using PET and Ĉerenkov imaging. Biomaterials. 2016;98:53–63. doi: 10.1016/j.biomaterials.2016.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kamkaew A, Cheng L, Goel S, et al. Cerenkov Radiation Induced Photodynamic Therapy Using Chlorin e6-Loaded Hollow Mesoporous Silica Nanoparticles. ACS Applied Materials & Interfaces. 2016;8:26630–26637. doi: 10.1021/acsami.6b10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee SB, Ahn SB, Lee SW, et al. Radionuclide-embedded gold nanoparticles for enhanced dendritic cell-based cancer immunotherapy, sensitive and quantitative tracking of dendritic cells with PET and Cerenkov luminescence. NPG Asia Materials. 2016;8:e281. [Google Scholar]
- 102.Lee SB, Yoon G, Lee SW, et al. Combined Positron Emission Tomography and Cerenkov Luminescence Imaging of Sentinel Lymph Nodes Using PEGylated Radionuclide-Embedded Gold Nanoparticles. Small. 2016;12:4894–4901. doi: 10.1002/smll.201601721. [DOI] [PubMed] [Google Scholar]
- 103.Hu Z, Qu Y, Wang K, et al. In vivo nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging. Nature Communications. 2015;6:7560. doi: 10.1038/ncomms8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thorek DL, Ogirala A, Beattie BJ, Grimm J. Quantitative imaging of disease signatures through radioactive decay signal conversion. Nat Med. 2013;19:1345–1350. doi: 10.1038/nm.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu R, Zhang G, Mai J, et al. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotech. 2016;34:414–418. doi: 10.1038/nbt.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang R, Fan Q, Yang M, et al. Engineering Melanin Nanoparticles as an Efficient Drug–Delivery System for Imaging-Guided Chemotherapy. Advanced Materials. 2015;27:5063–5069. doi: 10.1002/adma.201502201. [DOI] [PubMed] [Google Scholar]
- 107.Yan Y, Liu L, Xiong H, et al. Functional polyesters enable selective siRNA delivery to lung cancer over matched normal cells. Proc Natl Acad Sci U S A. 2016;113:E5702–5710. doi: 10.1073/pnas.1606886113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng D, Giljohann DA, Chen DL, et al. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proceedings of the National Academy of Sciences. 2012;109:11975–11980. doi: 10.1073/pnas.1118425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alidori S, Akhavein N, Thorek DLJ, et al. Targeted fibrillar nanocarbon RNAi treatment of acute kidney injury. Science Translational Medicine. 2016;8:331ra339. doi: 10.1126/scitranslmed.aac9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Near-Infrared Resonant Nanoshells for Combined Optical Imaging and Photothermal Cancer Therapy. Nano Letters. 2007;7:1929–1934. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 111.Stern JM, Stanfield J, Kabbani W, Hsieh JT, Cadeddu JA. Selective Prostate Cancer Thermal Ablation With Laser Activated Gold Nanoshells. The Journal of Urology. 179:748–753. doi: 10.1016/j.juro.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 112.Schwartz JA, Shetty AM, Price RE, et al. Feasibility Study of Particle-Assisted Laser Ablation of Brain Tumors in Orthotopic Canine Model. Cancer Research. 2009;69:1659. doi: 10.1158/0008-5472.CAN-08-2535. [DOI] [PubMed] [Google Scholar]
- 113.Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted Nanoshells for Integrated Cancer Imaging and Therapy. Nano Letters. 2005;5:709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 114.Cardinal J, Klune JR, Chory E, et al. Noninvasive radiofrequency ablation of cancer targeted by gold nanoparticles. Surgery. 144:125–132. doi: 10.1016/j.surg.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gannon CJ, Patra CR, Bhattacharya R, Mukherjee P, Curley SA. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. Journal of Nanobiotechnology. 2008;6:2. doi: 10.1186/1477-3155-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nano. 2015;10:370–379. doi: 10.1038/nnano.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat Med. 2012;18:1580–1585. doi: 10.1038/nm.2933. [DOI] [PubMed] [Google Scholar]
- 118.Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. Journal of Neuro-Oncology. 2011;103:317–324. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johannsen M, Gneveckow U, Taymoorian K, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. International Journal of Hyperthermia. 2007;23:315–323. doi: 10.1080/02656730601175479. [DOI] [PubMed] [Google Scholar]
- 120.van Landeghem FKH, Maier-Hauff K, Jordan A, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30:52–57. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 121.Hayashi K, Nakamura M, Sakamoto W, et al. Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics. 2013;3:366–376. doi: 10.7150/thno.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jordan A, Scholz R, Maier-Hauff K, et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. Journal of Neuro-Oncology. 2006;78:7–14. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- 123.Kalber TL, Ordidge KL, Southern P, et al. Hyperthermia treatment of tumors by mesenchymal stem cell-delivered superparamagnetic iron oxide nanoparticles. Int J Nanomedicine. 2016;11:1973–1983. doi: 10.2147/IJN.S94255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Timbie K, Nance E, Zhang C, et al. Ultrasound-mediated nanoparticle delivery across the blood-brain barrier (676.17) The FASEB Journal. 2014;28 [Google Scholar]
- 125.Rapoport N, Payne A, Dillon C, Shea J, Scaife C, Gupta R. Focused ultrasound-mediated drug delivery to pancreatic cancer in a mouse model. J Ther Ultrasound. 2013;1:11. doi: 10.1186/2050-5736-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morch Y, Hansen R, Berg S, et al. Nanoparticle-stabilized microbubbles for multimodal imaging and drug delivery. Contrast Media Mol Imaging. 2015;10:356–366. doi: 10.1002/cmmi.1639. [DOI] [PubMed] [Google Scholar]
- 127.Min HS, Son S, You DG, et al. Chemical gas-generating nanoparticles for tumor-targeted ultrasound imaging and ultrasound-triggered drug delivery. Biomaterials. 2016;108:57–70. doi: 10.1016/j.biomaterials.2016.08.049. [DOI] [PubMed] [Google Scholar]
- 128.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fadel TR, Sharp FA, Vudattu N, et al. A carbon nanotube–polymer composite for T-cell therapy. Nat Nano. 2014;9:639–647. doi: 10.1038/nnano.2014.154. [DOI] [PubMed] [Google Scholar]
- 131.McLaughlin MF, Robertson D, Pevsner PH, Wall JS, Mirzadeh S, Kennel SJ. LnPO4 nanoparticles doped with Ac-225 and sequestered daughters for targeted alpha therapy. Cancer biotherapy & radiopharmaceuticals. 2014;29:34–41. doi: 10.1089/cbr.2013.1546. [DOI] [PubMed] [Google Scholar]
- 132.McLaughlin MF, Woodward J, Boll RA, et al. Gold Coated Lanthanide Phosphate Nanoparticles for Targeted Alpha Generator Radiotherapy. PLOS ONE. 2013;8:e54531. doi: 10.1371/journal.pone.0054531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen J, Zhu S, Tong L, et al. Superparamagnetic iron oxide nanoparticles mediated (131)I-hVEGF siRNA inhibits hepatocellular carcinoma tumor growth in nude mice. BMC Cancer. 2014;14:114–114. doi: 10.1186/1471-2407-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klutz K, Schaffert D, Willhauck MJ, et al. Epidermal Growth Factor Receptor-targeted (131)I-therapy of Liver Cancer Following Systemic Delivery of the Sodium Iodide Symporter Gene. Molecular Therapy. 2011;19:676–685. doi: 10.1038/mt.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Satterlee AB, Yuan H, Huang L. A radio-theranostic nanoparticle with high specific drug loading for cancer therapy and imaging. Journal of Controlled Release. 2015;217:170–182. doi: 10.1016/j.jconrel.2015.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Das M, Datir SR, Singh RP, Jain S. Augmented Anticancer Activity of a Targeted, Intracellularly Activatable, Theranostic Nanomedicine Based on Fluorescent and Radiolabeled, Methotrexate-Folic Acid-Multiwalled Carbon Nanotube Conjugate. Molecular Pharmaceutics. 2013;10:2543–2557. doi: 10.1021/mp300701e. [DOI] [PubMed] [Google Scholar]
- 137.Zhang XD, Luo Z, Chen J, et al. Ultrasmall Au10–12(SG)10–12 Nanomolecules for High Tumor Specificity and Cancer Radiotherapy. Advanced Materials. 2014;26:4565–4568. doi: 10.1002/adma.201400866. [DOI] [PubMed] [Google Scholar]
- 138.Al Zaki A, Joh D, Cheng Z, et al. Gold-Loaded Polymeric Micelles for Computed Tomography-Guided Radiation Therapy Treatment and Radiosensitization. ACS Nano. 2014;8:104–112. doi: 10.1021/nn405701q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hainfeld JF, Smilowitz HM, O'Connor MJ, Dilmanian FA, Slatkin DN. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine (Lond) 2013;8:1601–1609. doi: 10.2217/nnm.12.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Popovtzer A, Mizrachi A, Motiei M, et al. Actively targeted gold nanoparticles as novel radiosensitizer agents: an in vivo head and neck cancer model. Nanoscale. 2016;8:2678–2685. doi: 10.1039/c5nr07496g. [DOI] [PubMed] [Google Scholar]
- 141.Liu P, Huang Z, Chen Z, et al. Silver nanoparticles: a novel radiation sensitizer for glioma? Nanoscale. 2013;5:11829–11836. doi: 10.1039/c3nr01351k. [DOI] [PubMed] [Google Scholar]
- 142.Moeendarbari S, Tekade R, Mulgaonkar A, et al. Theranostic Nanoseeds for Efficacious Internal Radiation Therapy of Unresectable Solid Tumors. Scientific Reports. 2016;6:20614. doi: 10.1038/srep20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yi X, Yang K, Liang C, et al. Imaging-Guided Combined Photothermal and Radiotherapy to Treat Subcutaneous and Metastatic Tumors Using Iodine-131-Doped Copper Sulfide Nanoparticles. Advanced Functional Materials. 2015;25:4689–4699. [Google Scholar]
- 144.Chen F, Rieffel J, Chen G, et al. Hexamodal imaging in vivo with nanoparticles. Journal of Nuclear Medicine. 2015;56:56–56. [Google Scholar]
- 145.Rieffel J, Chen F, Kim J, et al. Hexamodal Imaging with Porphyrin-Phospholipid-Coated Upconversion Nanoparticles. Advanced materials (Deerfield Beach, Fla) 2015;27:1785–1790. doi: 10.1002/adma.201404739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Conde J, Oliva N, Zhang Y, Artzi N. Local triple-combination therapy results in tumour regression and prevents recurrence in a colon cancer model. Nat Mater. 2016;15:1128–1138. doi: 10.1038/nmat4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kaittanis C, Shaffer TM, Bolaender A, et al. Multifunctional MRI/PET Nanobeacons Derived from the in Situ Self-Assembly of Translational Polymers and Clinical Cargo through Coalescent Intermolecular Forces. Nano Lett. 2015;15:8032–8043. doi: 10.1021/acs.nanolett.5b03370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kaittanis C, Shaffer TM, Ogirala A, et al. Environment-responsive nanophores for therapy and treatment monitoring via molecular MRI quenching. Nat Commun. 2014;5:3384. doi: 10.1038/ncomms4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miller MA, Gadde S, Pfirschke C, et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Science Translational Medicine. 2015;7:314ra183–314ra183. doi: 10.1126/scitranslmed.aac6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van de Ven AL, Abdollahi B, Martinez CJ, et al. Modeling of nanotherapeutics delivery based on tumor perfusion. New J Phys. 2013;15:55004. doi: 10.1088/1367-2630/15/5/055004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu L, Cai X, Nelson K, et al. A green synthesis of carbon nanoparticles from honey and their use in real-time photoacoustic imaging. Nano Research. 2013;6:312–325. doi: 10.1007/s12274-013-0308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sharma N, Pinnaka AK, Raje M, Fnu A, Bhattacharyya MS, Choudhury AR. Exploitation of marine bacteria for production of gold nanoparticles. Microb Cell Fact. 2012;11:86. doi: 10.1186/1475-2859-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kikuchi F, Kato Y, Furihata K, et al. Formation of gold nanoparticles by glycolipids of Lactobacillus casei. Sci Rep. 2016;6:34626. doi: 10.1038/srep34626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Parodi A, Quattrocchi N, van de Ven AL, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Braun GB, Friman T, Pang HB, et al. Etchable plasmonic nanoparticle probes to image and quantify cellular internalization. Nat Mater. 2014;13:904–911. doi: 10.1038/nmat3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim SE, Zhang L, Ma K, et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nano. 2016;11:977–985. doi: 10.1038/nnano.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li M, Li L, Zhan C, Kohane DS. Core-Shell Nanostars for Multimodal Therapy and Imaging. Theranostics. 2016;6:2306–2313. doi: 10.7150/thno.15843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen F, Ellison PA, Lewis CM, et al. Chelator-free synthesis of a dual-modality PET/MRI agent. Angewandte Chemie (International ed in English) 2013;52:13319–13323. doi: 10.1002/anie.201306306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chakravarty R, Valdovinos HF, Chen F, et al. Intrinsically germanium-69-labeled iron oxide nanoparticles: synthesis and in-vivo dual-modality PET/MR imaging. Adv Mater. 2014;26:5119–5123. doi: 10.1002/adma.201401372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Siddiqi KS, Ur Rahman A, Tajuddin, Husen A. Biogenic Fabrication of Iron/Iron Oxide Nanoparticles and Their Application. Nanoscale Res Lett. 2016;11:498. doi: 10.1186/s11671-016-1714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tafoya MA, Madi S, Sillerud LO. Superparamagnetic nanoparticle-enhanced MRI of Alzheimer's disease plaques and activated microglia in 3X transgenic mouse brains: Contrast optimization. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25563. [DOI] [PubMed] [Google Scholar]
- 162.Madru R, Tran TA, Axelsson J, et al. (68)Ga-labeled superparamagnetic iron oxide nanoparticles (SPIONs) for multi-modality PET/MR/Cherenkov luminescence imaging of sentinel lymph nodes. American Journal of Nuclear Medicine and Molecular Imaging. 2013;4:60–69. [PMC free article] [PubMed] [Google Scholar]
- 163.Aghighi M, Golovko D, Ansari C, et al. Imaging Tumor Necrosis with Ferumoxytol. PLoS One. 2015;10:e0142665. doi: 10.1371/journal.pone.0142665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Khurana A, Chapelin F, Beck G, et al. Iron administration before stem cell harvest enables MR imaging tracking after transplantation. Radiology. 2013;269:186–197. doi: 10.1148/radiol.13130858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sciallero C, Balbi L, Paradossi G, Trucco A. Magnetic resonance and ultrasound contrast imaging of polymer-shelled microbubbles loaded with iron oxide nanoparticles. R Soc Open Sci. 2016;3:160063. doi: 10.1098/rsos.160063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nissinen T, Nakki S, Laakso H, et al. Tailored Dual PEGylation of Inorganic Porous Nanocarriers for Extremely Long Blood Circulation in Vivo. ACS Appl Mater Interfaces. 2016;8:32723–32731. doi: 10.1021/acsami.6b12481. [DOI] [PubMed] [Google Scholar]
- 167.Cui Y, Zhang C, Luo R, et al. Noninvasive monitoring of early antiangiogenic therapy response in human nasopharyngeal carcinoma xenograft model using MRI with RGD-conjugated ultrasmall superparamagnetic iron oxide nanoparticles. Int J Nanomedicine. 2016;11:5671–5682. doi: 10.2147/IJN.S115357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jaidev LR, Chellappan DR, Bhavsar DV, et al. Multi-functional nanoparticles as theranostic agents for the treatment & imaging of pancreatic cancer. Acta Biomater. 2016 doi: 10.1016/j.actbio.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 169.Liu Q, Sun Y, Li C, et al. 18F-Labeled magnetic-upconversion nanophosphors via rare-Earth cation-assisted ligand assembly. ACS Nano. 2011;5:3146–3157. doi: 10.1021/nn200298y. [DOI] [PubMed] [Google Scholar]
- 170.Xiang Z, Yang X, Xu J, et al. Tumor detection using magnetosome nanoparticles functionalized with a newly screened EGFR/HER2 targeting peptide. Biomaterials. 2017;115:53–64. doi: 10.1016/j.biomaterials.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 171.Wang Y, Liu Y, Luehmann H, et al. Radioluminescent gold nanocages with controlled radioactivity for real-time in vivo imaging. Nano Lett. 2013;13:581–585. doi: 10.1021/nl304111v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Perez-Campana C, Gomez-Vallejo V, Puigivila M, et al. Biodistribution of different sized nanoparticles assessed by positron emission tomography: a general strategy for direct activation of metal oxide particles. ACS Nano. 2013;7:3498–3505. doi: 10.1021/nn400450p. [DOI] [PubMed] [Google Scholar]
- 173.Perez-Campana C, Gomez-Vallejo V, Martin A, et al. Tracing nanoparticles in vivo: a new general synthesis of positron emitting metal oxide nanoparticles by proton beam activation. Analyst. 2012;137:4902–4906. doi: 10.1039/c2an35863h. [DOI] [PubMed] [Google Scholar]
- 174.Zhou M, Zhang R, Huang M, et al. A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J Am Chem Soc. 2010;132:15351–15358. doi: 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thorek DLJ, Das S, Grimm J. Molecular Imaging Using Nanoparticle Quenchers of Cerenkov Luminescence. Small. 2014;10:3729–3734. doi: 10.1002/smll.201400733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lin X, Xie J, Niu G, et al. Chimeric ferritin nanocages for multiple function loading and multimodal imaging. Nano Lett. 2011;11:814–819. doi: 10.1021/nl104141g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Spaliviero M, Harmsen S, Huang R, et al. Detection of Lymph Node Metastases with SERRS Nanoparticles. Molecular Imaging and Biology. 2016;18:677–685. doi: 10.1007/s11307-016-0932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mead BP, Mastorakos P, Suk JS, Klibanov AL, Hanes J, Price RJ. Targeted gene transfer to the brain via the delivery of brain-penetrating DNA nanoparticles with focused ultrasound. J Control Release. 2016;223:109–117. doi: 10.1016/j.jconrel.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ma X, Kang F, Xu F, et al. Enhancement of Cerenkov Luminescence Imaging by Dual Excitation of Er3+, Yb3+-Doped Rare-Earth Microparticles. PLoS ONE. 2013;8:e77926. doi: 10.1371/journal.pone.0077926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhenhua H, Liu M, Guo H, Zhang Z, Tian J. Image-guided cancer surgery using a novel nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging. Journal of Nuclear Medicine. 2016;57:59–59. [Google Scholar]
- 181.Lee BR, Ko HK, Ryu JH, et al. Engineered Human Ferritin Nanoparticles for Direct Delivery of Tumor Antigens to Lymph Node and Cancer Immunotherapy. Scientific Reports. 2016;6:35182. doi: 10.1038/srep35182. [DOI] [PMC free article] [PubMed] [Google Scholar]


