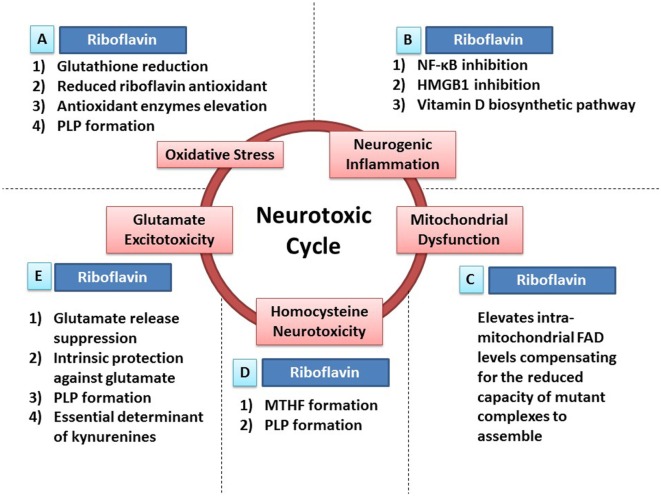
Figure 1.
Riboflavin protects against neurotoxicity through ameliorating oxidative stress, mitochondrial dysfunction, neurogenic inflammation, glutamate excitotoxicity, and homocysteine neurotoxicity. Oxidative stress, mitochondrial dysfunction, neurogenic inflammation, glutamate excitotoxicity, and homocysteine neurotoxicity are involved in neurodegeneration and neurotoxicity. Also, those neurotoxic factors have the ability to cause each other leading to the formation of a neurotoxic cycle. Riboflavin is capable of attacking this proposed neurotoxic cycle via multiple neuroprotective mechanisms that tackle different neurotoxic factors in this neurotoxic cycle. (A) In fact, riboflavin attacks oxidative stress via its antioxidant potential. First, glutathione reductase requires riboflavin for its action to reduce oxidized glutathione increasing the levels of reduced (active) glutathione. Second, riboflavin has independent antioxidant action through its reduced form (dihydroriboflavin). Third, riboflavin has the ability to elevate antioxidant enzymes levels such as SOD and catalase. Fourth, riboflavin is required for the formation of pyridoxal phosphate (PLP), the active vitamin B6, which has its own antioxidant activity (see Riboflavin Is Required for the Formation of Pyridoxal Phosphate). (B) In addition, riboflavin attacks neurogenic inflammation either directly or indirectly. Riboflavin has the ability to inhibit NF-κB and high-mobility group protein B1 (HMGB1), nuclear factors involved in inflammatory processes, demonstrating its direct anti-inflammatory activity. On the other hand, multiple enzymes in the biosynthetic pathway of vitamin D are riboflavin-dependent enzymes, thus, riboflavin exerts its indirect anti-inflammatory activity via its essential role in vitamin D synthesis, which has a potent anti-inflammatory activity. (C) Furthermore, administration of riboflavin is capable of elevating the intra-mitochondrial levels of flavin adenine dinucleotide (FAD), which will compensate for the reduced capacity of dysfunctional complexes to assemble. As a result, riboflavin aims to normalize mitochondrial function in dysfunctional states. (D) Moreover, elevated homocysteine levels exhibit neurotoxic effects. Riboflavin-dependent enzymes are critical steps in the synthesis of methyltetrahydrofolate (MTHF) and PLP. MTHF and PLP are required for the actions of homocysteine metabolizing enzymes; methionine synthase and cystathionine b-synthase, respectively (see Riboflavin Is Required for Homocysteine Metabolism). (E) Additionally, riboflavin has the ability to attack glutamate excitotoxicity. In fact, riboflavin inhibits the endogenous neuronal release of glutamate reducing its excitotoxicity potential. In addition, both riboflavin and PLP (riboflavin is required for its synthesis) have their intrinsic protective properties against glutamate toxicity by increasing the survival of neurons exposed to glutamate toxicity after being treated with riboflavin or PLP. Also, both riboflavin and PLP are essential determinants of the tryptophan–kynurenine pathway, which produce neuroactive compounds known as kynurenines that influences glutamate receptors, hence, modulating glutamate excitotoxicity potential (see Riboflavin as a Determinant of the Kynurenine Pathway and Riboflavin Can Ameliorate Glutamate Toxicity; Which Is Implicated in Parkinson’s Disease and Migraine).