Figure 1.
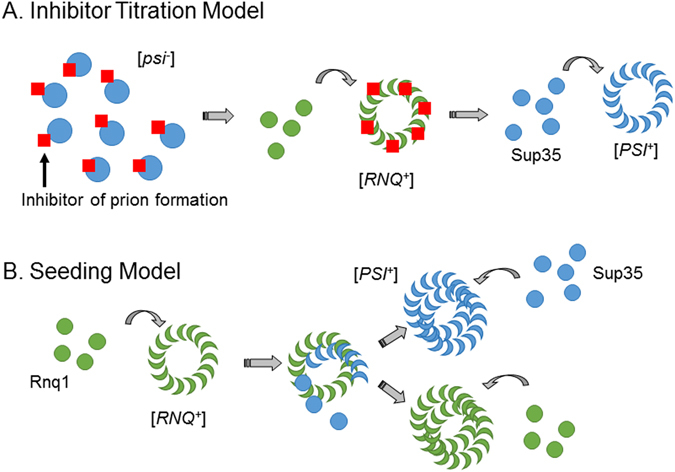
Models for the [RNQ +]-dependent formation of the [PSI +] prion. (A) The inhibitor titration model suggests that an inhibitor molecule (red squares) binds to Sup35 in its soluble state (blue circles). The presence of [RNQ +] (green ring) sequesters the inhibitor away from Sup35, thereby allowing the protein to aggregate and form [PSI +]. (B) The seeding model suggests that there is a physical interaction between Sup35 and aggregated Rnq1 during the formation of [PSI +]. After the interaction, the [RNQ +] and [PSI +] prions are propagated independently.
