Figure 3.
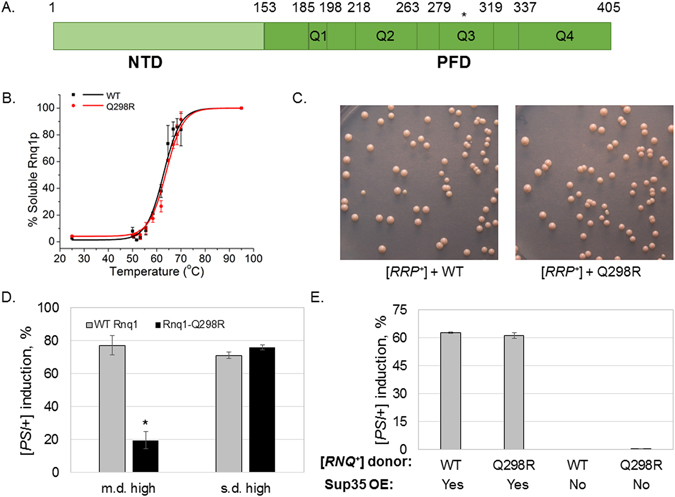
Rnq1-Q298R causes a [PSI +] induction defect. (A) The Rnq1 protein contains an N-terminal domain (NTD) and a glutamine/asparagine-rich prion-forming domain (PFD). The PFD contains four glutamine-dense regions, Q1-436. The Q298R mutation occurs in region Q3 of the PFD. (B) Rnq1 aggregates were treated at a gradient of temperatures in SDS to determine the melting point of the Rnq1-Q298R aggregates versus the WT. There were no significant differences in the thermostable properties of either protein aggregate. Western blot signal from multiple experiments was quantified using ImageJ, normalized by the 95C band, and plotted using Origin 9.0. Error bars represent standard error of the mean (s.e.m). (C) There are no detectable differences in mitotic stability of Rnq1-Q298R aggregates as compared to WT Rnq1 aggregates. [RNQ +] strains containing the [R NQ +] Reporter Protein (RRP), a phenotypic readout for the [RNQ +] prion37, were transformed with plasmids harboring either WT RNQ1 or rnq1-Q298R. We assessed the mitotic stability, or spontaneous prion loss, of resulting strains and found that [RNQ +] formed from WT or Rnq1-Q298R was similarly maintained. (D) The rnq1-Q298R mutant shows a strong defect in [PSI +] induction relative to WT cells of the m.d. high variant of [RNQ +]. [PSI +] was induced by overexpression of Sup35 in [psi −][RNQ +] cells of either a RNQ1 or rnq1-Q298R genetic background. Colonies were assessed by color, with white or pink colonies or sectored colonies scored as [PSI +]. Error bars represent mean ± s.e.m. The “*” symbol represents a significant difference between [PSI +] induction in RNQ1 versus rnq1-Q298R backgrounds, p < 0.001. (E) Results from [PSI +] induction following cytoplasmic transfer of either WT Rnq1 or Rnq1-Q298R protein aggregates into a [rnq −] RNQ1 strain. The propagated [RNQ +] would be templated from the WT or mutant aggregate, but be comprised of only WT protein. Error bars represent mean ± s.e.m.
