FIG 1 .
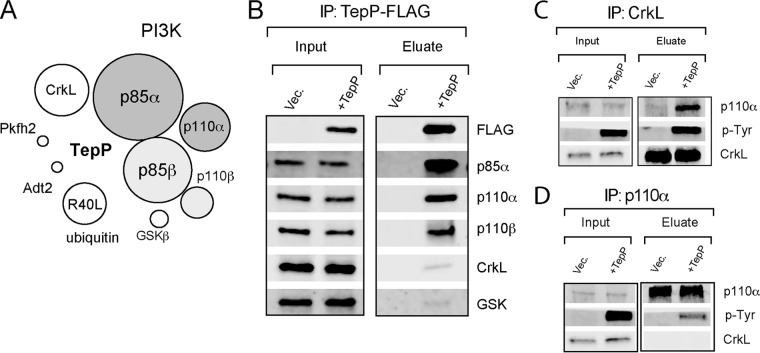
TepP forms complexes with PI3K and CrkL in infected cells. (A) Schematic representation of epithelial proteins that associate with TepP during infection. A2EN cells were infected with C. trachomatis expressing TepP-FLAG for 4 h, and cell lysates were subjected to immunoprecipitation (IP) with anti-FLAG antibodies. Bound proteins were digested with trypsin, and the resulting peptides were identified by mass spectrometry (MS). Sizes of circles correspond to the relative numbers of peptides identified for each protein by LC-MS/MS. (B) Immunoblot validation of TepP-interacting proteins. A2EN cells were infected for 4 h with CTLM062G1 transformed with a TepP-FLAG expression plasmid or vector-only control (Vec.), and cell lysates were subjected to IP with anti-FLAG antibodies. Bound proteins were detected by immunoblotting with specific antibodies. (C and D) Reciprocal co-IP of CrkL (C), PI3K (D), and TepP. A2EN cells were infected with CTLM062G1 strains as described for panel B, and at 4 h postinfection, CrkL and p110α were subjected to IP. Co-IP of proteins was assessed by immunoblot analysis.