Abstract
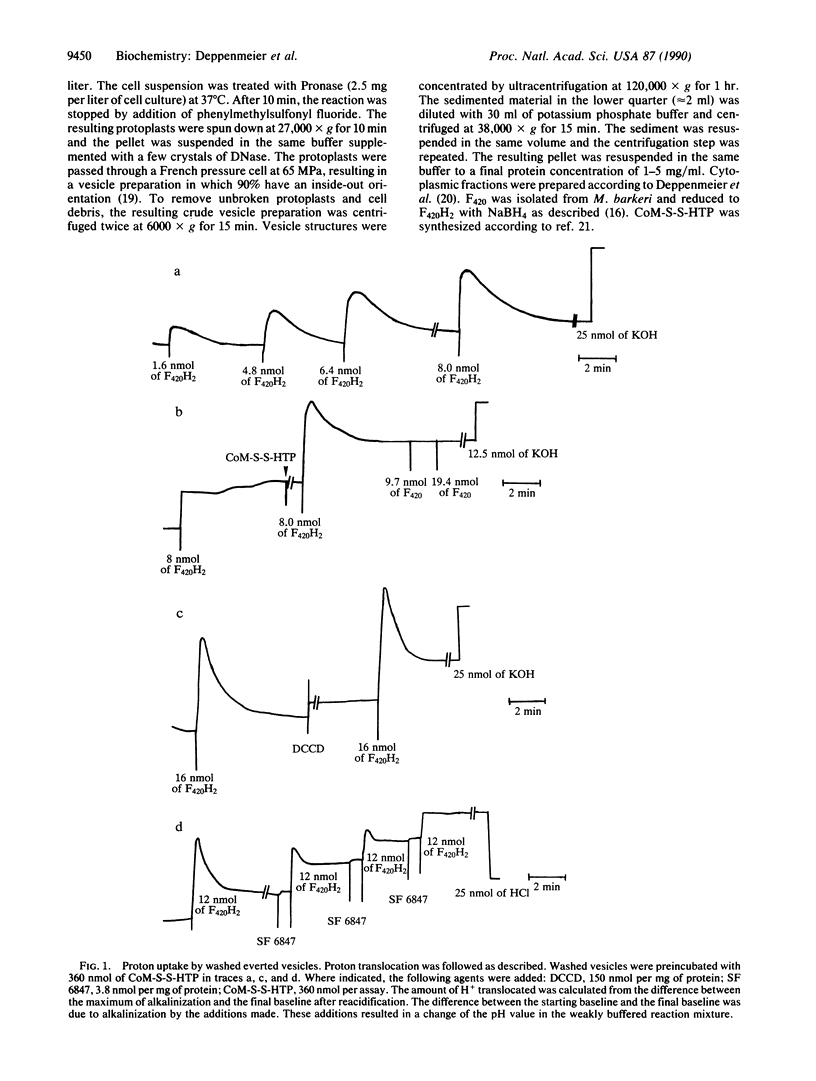
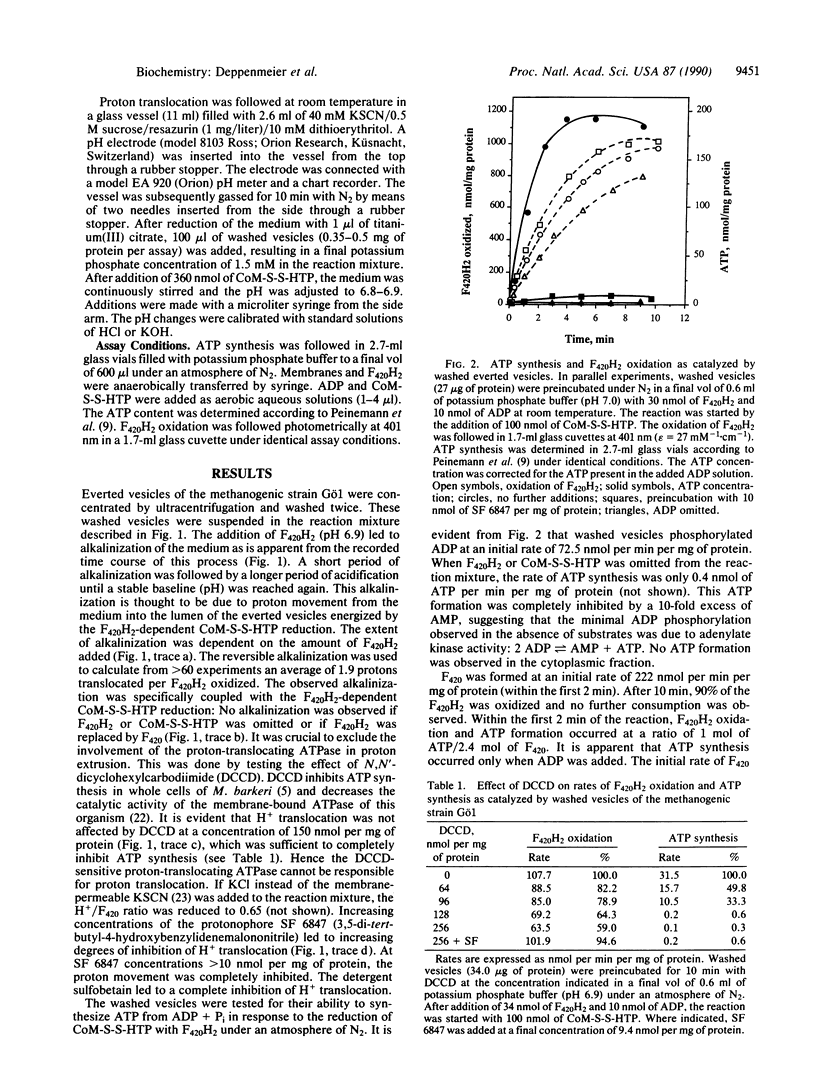
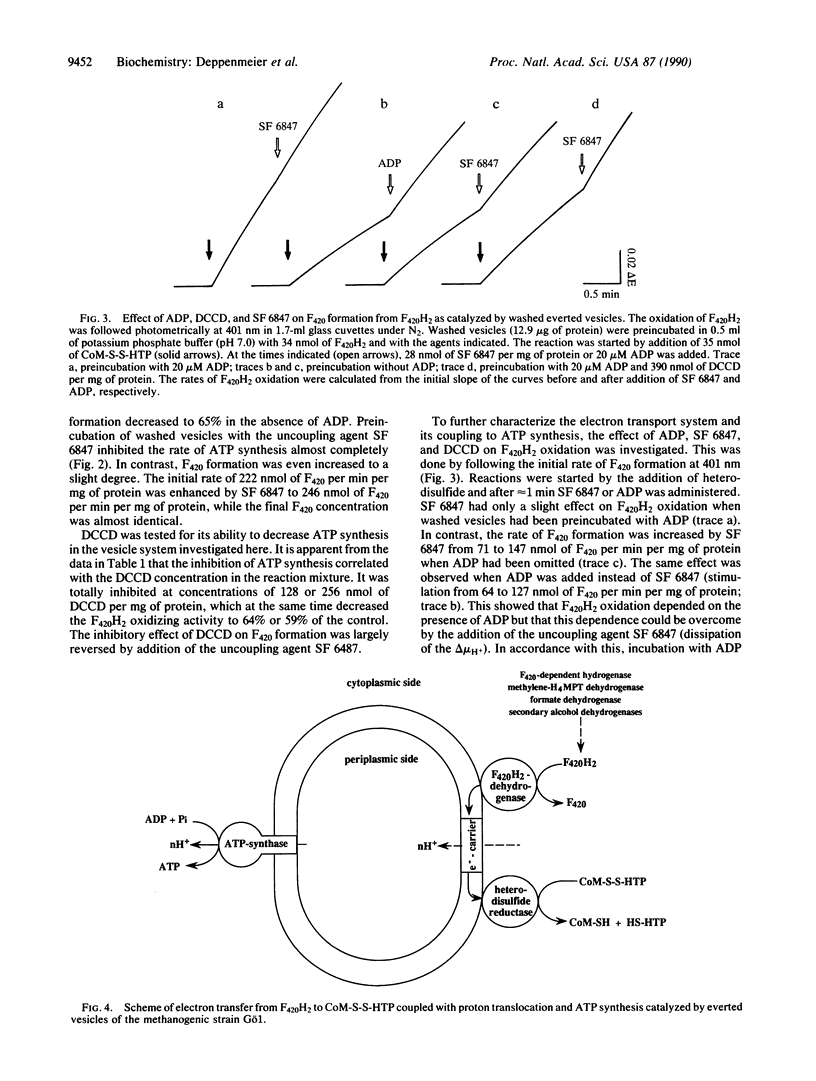
Washed everted vesicles of the methanogenic bacterium strain Go1 were found to couple the F420H2-dependent heterodisulfide reduction with the transfer of protons across the membrane into the lumen of the everted vesicles. The transmembrane electrochemical potential of protons thereby generated was shown to be competent in driving ATP synthesis from ADP + Pi, exhibiting a stoichiometry of 2 H+ translocated or 0.4 ATP synthesized per F420H2 oxidized. This enzyme system exhibits the phenomenon of coupling and uncoupling and represents a different kind of electron transport chain with the heterodisulfide of 2-mercaptoethanesulfonate and 7-mercaptoheptanoylthreonine phosphate as terminal electron acceptor. The heterodisulfide and methane are formed in the methyl coenzyme M reductase reaction. The reducing equivalents are derived from reduced coenzyme F420, which represents an analogue of NADH + H+ in other respiratory chains. It is assumed that the proton-translocating oxidoreductase discovered in strain Go1 is of principal importance to all methanogenic bacteria not utilizing H2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaut M., Gottschalk G. Coupling of ATP synthesis and methane formation from methanol and molecular hydrogen in Methanosarcina barkeri. Eur J Biochem. 1984 May 15;141(1):217–222. doi: 10.1111/j.1432-1033.1984.tb08178.x. [DOI] [PubMed] [Google Scholar]
- Bobik T. A., Olson K. D., Noll K. M., Wolfe R. S. Evidence that the heterodisulfide of coenzyme M and 7-mercaptoheptanoylthreonine phosphate is a product of the methylreductase reaction in Methanobacterium. Biochem Biophys Res Commun. 1987 Dec 16;149(2):455–460. doi: 10.1016/0006-291x(87)90389-5. [DOI] [PubMed] [Google Scholar]
- Deppenmeier U., Blaut M., Gottschalk G. Dependence on membrane components of methanogenesis from methyl-CoM with formaldehyde or molecular hydrogen as electron donors. Eur J Biochem. 1989 Dec 8;186(1-2):317–323. doi: 10.1111/j.1432-1033.1989.tb15211.x. [DOI] [PubMed] [Google Scholar]
- Deppenmeier U., Blaut M., Jussofie A., Gottschalk G. A methyl-CoM methylreductase system from methanogenic bacterium strain Gö 1 not requiring ATP for activity. FEBS Lett. 1988 Dec 5;241(1-2):60–64. doi: 10.1016/0014-5793(88)81031-7. [DOI] [PubMed] [Google Scholar]
- Ellermann J., Hedderich R., Böcher R., Thauer R. K. The final step in methane formation. Investigations with highly purified methyl-CoM reductase (component C) from Methanobacterium thermoautotrophicum (strain Marburg). Eur J Biochem. 1988 Mar 15;172(3):669–677. doi: 10.1111/j.1432-1033.1988.tb13941.x. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. Resolution and properties of the components. J Biol Chem. 1980 Mar 10;255(5):1891–1895. [PubMed] [Google Scholar]
- Hrúzik J., Kotuliaková M., Faybík M., Mayer V., Procházka M., Nádvorník P., Valach A. Herpetická encefalitída. Cas Lek Cesk. 1986 Feb 21;125(8):245–249. [PubMed] [Google Scholar]
- Inatomi K., Maeda M., Futai M. Dicyclohexylcarbodiimide-binding protein is a subunit of the Methanosarcina barkeri ATPase complex. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1585–1590. doi: 10.1016/0006-291x(89)90856-5. [DOI] [PubMed] [Google Scholar]
- Kühn W., Gottschalk G. Characterization of the cytochromes occurring in Methanosarcina species. Eur J Biochem. 1983 Sep 1;135(1):89–94. doi: 10.1111/j.1432-1033.1983.tb07621.x. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr Soluble and membrane-bound paramagnetic centers in methanobacterium bryantii. FEBS Lett. 1980 Jun 30;115(2):285–288. doi: 10.1016/0014-5793(80)81188-4. [DOI] [PubMed] [Google Scholar]
- Mayer F., Jussofie A., Salzmann M., Lübben M., Rohde M., Gottschalk G. Immunoelectron microscopic demonstration of ATPase on the cytoplasmic membrane of the methanogenic bacterium strain Göl. J Bacteriol. 1987 May;169(5):2307–2309. doi: 10.1128/jb.169.5.2307-2309.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll K. M., Donnelly M. I., Wolfe R. S. Synthesis of 7-mercaptoheptanoylthreonine phosphate and its activity in the methylcoenzyme M methylreductase system. J Biol Chem. 1987 Jan 15;262(2):513–515. [PubMed] [Google Scholar]
- Noll K. M., Rinehart K. L., Jr, Tanner R. S., Wolfe R. S. Structure of component B (7-mercaptoheptanoylthreonine phosphate) of the methylcoenzyme M methylreductase system of Methanobacterium thermoautotrophicum. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4238–4242. doi: 10.1073/pnas.83.12.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinemann S., Blaut M., Gottschalk G. ATP synthesis coupled to methane formation from methyl-CoM and H2 catalyzed by vesicles of the methanogenic bacterial strain Gö1. Eur J Biochem. 1989 Dec 8;186(1-2):175–180. doi: 10.1111/j.1432-1033.1989.tb15192.x. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Beckler G. S., Cram D. S., Hamilton P. T., Brown J. W., Krzycki J. A., Kolodziej A. F., Alex L., Orme-Johnson W. H., Walsh C. T. A hydrogenase-linked gene in Methanobacterium thermoautotrophicum strain delta H encodes a polyferredoxin. Proc Natl Acad Sci U S A. 1989 May;86(9):3031–3035. doi: 10.1073/pnas.86.9.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière P. E., Wolfe R. S. Novel biochemistry of methanogenesis. J Biol Chem. 1988 Jun 15;263(17):7913–7916. [PubMed] [Google Scholar]
- Shapiro S., Wolfe R. S. Methyl-coenzyme M, an intermediate in methanogenic dissimilation of C1 compounds by Methanosarcina barkeri. J Bacteriol. 1980 Feb;141(2):728–734. doi: 10.1128/jb.141.2.728-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meijden P., Heythuysen H. J., Pouwels A., Houwen F., van der Drift C., Vogels G. D. Methyltransferases involved in methanol conversion by Methanosarcina barkeri. Arch Microbiol. 1983 Jun;134(3):238–242. doi: 10.1007/BF00407765. [DOI] [PubMed] [Google Scholar]


