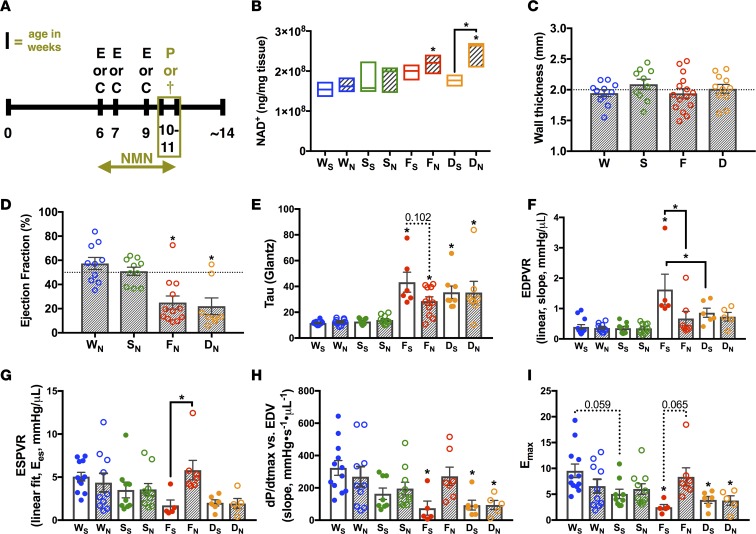
Figure 3. NMN in the FXN-KO improves diastolic and normalizes systolic function in a SIRT3-dependent manner.
(A) Experimental schedule of treatment and clinical phenotyping protocols. E, echocardiography; C, noninvasive monitoring by CLAMS; P, PV-loop analysis; and †, sacrifice and collection of tissues for further analysis. Terminal procedures represented by olive-colored box. (B) Metabolomics profiling of cardiac NAD+ levels. Values are means ± SEM (n = 3–5 mice/group). (C) Wall thickness in NMN-treated animals measured via echocardiography at 9–10 weeks of age. Values are means ± SEM (n = 7–12 mice/group). (D–F) Hemodynamic measures assessed by PV-loop analysis: ejection fraction (D), active relaxation (τ, E), passive filling (linear end diastolic pressure volume relation [EDPVR], F), and contractility (linear ESPVR, G; dP/dtmax vs. end-diastolic volume [EDV], H; maximal elastance [Emax], I). Values are means ± SEM (E and G–I, n = 5–10 mice/group; D and F, n = 7–10 mice/group). Hashed line at 50% represents normal mouse ejection fraction. *P < 0.05, difference from saline-treated WT as determined by two-way ANOVA for 8 groups (4 genotypes, 2 treatment) and Bonferroni correction. W, WT; F, FXN-KO; S, SIRT3-KO; D, dKO; subscript S, saline; and subscript N, nicotinamide mononucleotide (NMN).