Abstract
Higher diet-dependent nonvolatile acid load is associated with faster chronic kidney disease (CKD) progression, but most studies have used estimated acid load or measured only components of the gold-standard, net acid excretion (NAE). Here we measured NAE as the sum of urine ammonium and titratable acidity in 24 hour urines from a random subset of 980 participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. In multivariable models accounting for demographics, comorbidity and kidney function, higher NAE was significantly associated with lower serum bicarbonate (0.17 mEq/L lower serum bicarbonate per 10 mEq/day higher NAE), consistent with a larger acid load. Over a median of 6 years follow-up, higher NAE was independently associated with a significantly lower risk of the composite of end stage renal disease or halving of estimated glomerular filtration rate among diabetics (hazard ratio 0.88 per 10 mEq/day higher NAE), but not those without diabetes (hazard ratio 1.04 per 10 mEq/day higher NAE). For comparison, we estimated nonvolatile acid load as net endogenous acid production using self-reported food frequency questionnaires from 2,848 patients and dietary urine biomarkers from 3,385 patients. Higher net endogenous acid production based on biomarkers (urea nitrogen and potassium) was modestly associated with faster CKD progression consistent with prior reports, but only among those without diabetes. Results from the food frequency questionnaires were not associated with CKD progression in any group. Thus, disparate results obtained from analyses of nonvolatile acid load directly measured as NAE and estimated from diet, suggests a novel hypothesis, that the risk of CKD progression related to low NAE, or acid load, may be due to diet-independent changes in acid production in diabetes.
Keywords: Chronic kidney disease, diabetic nephropathy, nutrition
Introduction
Metabolic acidosis is a modifiable risk factor for progression of chronic kidney disease (CKD).1-3 Treatment of CKD patients with sodium bicarbonate slowed progression in small randomized studies,4,5 but it remains unclear whether sodium bicarbonate exerts its beneficial effects by raising systemic pH, or by lowering the amount of acid that must be excreted in the urine, also known as the nonvolatile acid load.6 Prior studies suggest that higher nonvolatile acid load is associated with progression of CKD; however those studies relied on estimated acid load from dietary data rather than direct measurements, and many focused exclusively on patients with hypertensive kidney disease.7-9 Since patients with diabetes exhibit differences in urinary acidification and acid production compared to those without diabetes,10 the clinical impact of acid load on CKD progression may also differ in patients with diabetic nephropathy versus other forms of CKD.
Excreting the load of nonvolatile acids generated during metabolism is a critical homeostatic function of the kidneys. Nonvolatile acids (i.e. H+) are produced when: 1) sulfur-containing amino acids are oxidized to inorganic sulfate, and 2)the conjugate base of endogenously produced organic acids (e.g. citric acid) are excreted in the urine as an organic anion salt (e.g. sodium citrate).11-13 Alkali may be ingested in the form of alkali supplements or metabolizable organic anion salts found abundantly in fruits and vegetables, both of which may buffer nonvolatile acids, at least in part.9,14,15 Thus, the net load of nonvolatile acid that must be excreted by the kidney equals the difference between acids produced and alkali consumed in foods or supplements. The kidney excretes the nonvolatile acid load as ammonium (NH4+) or as titratable acid bound to anionic urinary buffers, such as phosphate and creatinine.16,17 Regulation of acid excretion and production maintains acid-base homeostasis in response to dietary changes or systemic acid-base perturbations.18,19
Since the daily net acid excretion approximates the daily nonvolatile acid load in the steady state, direct measurement of 24-hour urinary net acid excretion is the reference standard for measuring acid load.20 However, the relationship between 24-hour urine net acid excretion and renal outcomes in patients with CKD remains unknown. We hypothesized a priori that higher 24-hour net acid excretion would be associated with higher risk of CKD progression overall and particularly among those with diabetes since they have a greater predisposition to metabolic acidosis.21 To test this hypothesis, we directly measured net acid excretion in participants from the Chronic Renal Insufficiency Cohort Study (CRIC), a diverse CKD population including approximately equal number of patients with and without diabetes. Additionally, prior investigations have questioned whether nonvolatile acid load truly equals acid excretion in the setting of CKD.22-24 Thus, for comparison, we also estimated nonvolatile acid load from self-reported dietary intake data similar to prior reports.15
Results
Physiology of Acid Production and Excretion in CKD
We measured acid excretion in baseline 24-hour urine collections obtained in a randomly selected sample of 1000 participants from the Chronic Renal Insufficiency Cohort (CRIC) Study. We calculated net acid excretion as the sum of urinary ammonium and titratable acidity, calculated from urinary pH, phosphorus and creatinine. Nineteen urine samples with a pH ≥7.4 were excluded from further analysis due to concern for bacterial overgrowth. The ratio of urinary ammonium to urinary sulfate, a biomarker of acids produced from metabolism of sulfur-containing amino acids, was dramatically increased in these specimens (median 3.1 vs. 0.6 in urines with pH ≥7.4 compared with <7.4; p<0.01) suggesting exogenous ammonia production from urease positive bacteria. One additional sample had a urine pH value <4.0 that was deemed implausible, and therefore, was also excluded.
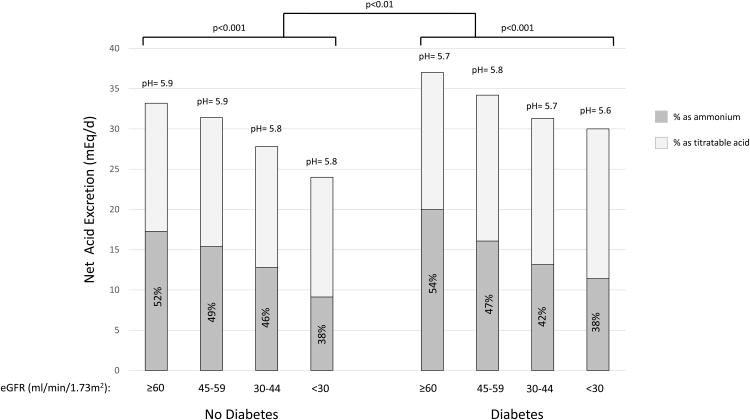
Characteristics of this subcohort of 980 individuals are similar to overall characteristics of the full CRIC cohort as previously published including mean age of 58 years, mean estimated glomerular filtration rate (eGFR) of 44 ml/min/1.73m2 and a population including 43% females, 41% non-Hispanic whites and 51% of participants with diabetes.25 Mean (± standard deviation) net acid excretion was 33 ± 18 mEq/day (Supplemental Figure 1). The percentage of acid excreted as ammonium was 46% ± 17%. Total net acid excretion and the percentage of acid excreted as ammonium was lower with lower eGFR (Figure 1a). Participants with diabetes had overall higher acid excretion, lower urine pH and lower percentage of acid excreted as ammonium compared to those without diabetes (each p<0.05). Participants with diabetes consumed more dietary protein as estimated by food frequency questionnaire (p<0.01), which may, in part, explain their higher acid excretion.
Figure 1. Median levels of acid excretion and markers of acid production by categories of eGFRand diabetes.
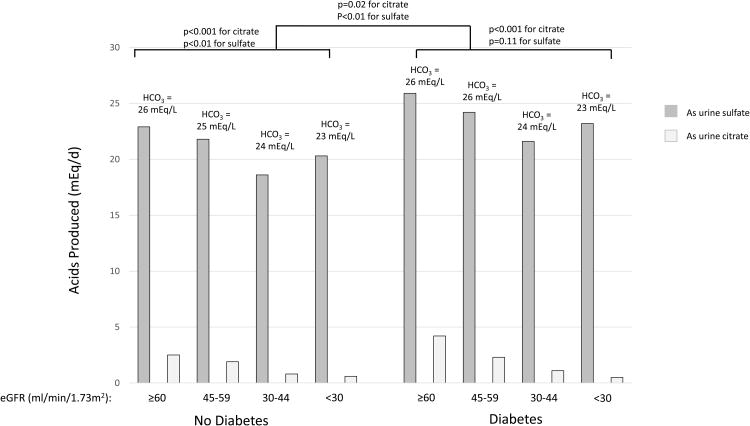
Estimated GFR is grouped according to Kidney Disease: Improving Global Outcomes (KDIGO) categorizations as G2 (eGFR 60-90 ml/min/1.73m2); G3a (eGFR 45-59 ml/min/1.73m2); G3b (eGFR 30-44 ml/min/1.73m2) and G4/5 (eGFR<30 ml/min/1.73m2).52 A) Median net acid excretion is depicted in mEq/day with the median percentage as ammonium depicted by dark gray bars. Urine ammonium was directly measured. Titratable acidity was calculated from urine pH, urinary phosphate and creatinine using the Henderson Hasselbalch equation.41 Median urine pH in each group is reported in text above the bars. P-values for difference in net acid excretion by diabetes status and kidney function are depicted above each set of bars. P-value for comparisons by diabetes are adjusted for eGFR. Overall, percentage of acid excretion as ammonium and urine pH each were lower at lower eGFR (each p value for trend<0.001 using a continuous linear model) and among patients with diabetes compared to those without (p=0.04 and 0.002, respectively). B) Biomarkers of acid production are depicted in mEq/day. Urine sulfate (dark gray bars) represents equimolar amounts of acid produced during metabolism of organic sulfur. Urine citrate represents total urine citrate in mEq based on each participants' urine pH (light gray bars). Thus this represents amount of acid produced from intermediate metabolism with losses of citrate salts in the urine. Citrate represents only one potential organic anion in the urine, whereas total organic anion excretion is estimated at 0.5mEq/kg/day;18 therefore, total acid production is not depicted. Median serum bicarbonate concentration (HCO3-) in each group is reported above the bars. P-values for difference in net acid excretion by diabetes status and kidney function are depicted above each set of bars. P-value for comparisons by diabetes are adjusted for eGFR. Overall, serum bicarbonate, urine citrate and urine sulfate were lower at lower eGFR (p value for trend<0.01 for each using a continuous linear model).
Although net acid excretion should approximate nonvolatile acid load in steady state, this concept has been questioned in CKD with some studies suggesting daily acid retention due to net acid excretion that is lower than diet-dependent acid load.22-24 Therefore, we evaluated the relationship between acid excretion and objective markers of acid production, including urine sulfate and citrate. Milliequivalents (mEq) of sulfate in the urine represent an equal amount of acids produced during the metabolism of organic sulfur in dietary protein. Similarly, mEq of urinary organic anion salts represent equal amounts of acids net produced by endogenous generation of organic acids in metabolic processes including the tricarboxylic acid cycle. Overall, urine citrate and sulfate were lower in participants with lower eGFR (Figure 1b; each p<0.01 from a continuous linear model), but both were higher in participants with diabetes independent of eGFR (each p<0.05).
Clinical Determinants of Acid Excretion in CKD
Clinical characteristics according to quartiles of net acid excretion are reported in Table 1. In these univariate analyses, higher net acid excretion associated with male sex, white race, greater body size and eGFR, and lower serum potassium, among other variables (Table 1). Consistent with the effect of diet on nonvolatile acid load, higher quartiles of net acid excretion also associated with higher caloric intake and higher estimates of acid load from dietary data (each p<0.01; Table 1). After standardization to a 2000 kcal diet, higher acid excretion was associated with greater relative intake of animal proteins, which are known sources of acid, and lesser relative intake of fruits and vegetables, which are known sources of base (Table 1).
Table 1. Clinical and dietary characteristics by quartiles of measured net acid excretion.
| Quartile of Net acid excretion (mEq/d) | |||||
|---|---|---|---|---|---|
| Characteristic in N (%) or mean ± SD | 1 (<20.4) N=245 |
2 (20.4–30.7) N=243 |
3 (30.8–41.8) N=246 |
4 (≥41.9) N=246 |
P* |
| Demographics | |||||
| Age (years) | 59 ±11 | 59 ±11 | 58 ±12 | 56 ±10 | 0.02 |
| Female sex | 140 (57%) | 125 (51%) | 95 (39%) | 66 (27%) | <0.01 |
| Race | <0.01 | ||||
| Non-Hispanic white | 70 (29%) | 82 (34%) | 114 (46%) | 133 (54%) | |
| Non-Hispanic black | 128 (52%) | 117 (48%) | 95 (39%) | 85 (35%) | |
| Hispanic | 39 (16%) | 37 (15%) | 26 (11%) | 23 (9%) | |
| Other | 8 (3%) | 7 (3%) | 11 (4%) | 5 (2%) | |
| Medical History | |||||
| Diabetes | 116 (47%) | 113 (47%) | 132 (54%) | 136 (55%) | 0.13 |
| Cardiovascular disease | 88 (36%) | 70 (29%) | 67 (27%) | 87 (35%) | 0.08 |
| Exam and Laboratory | |||||
| Body mass index (kg/m2) | 29.9 ± 8.3 | 31.5 ± 7.6 | 33.5 ± 8.5 | 33.7 ± 7.2 | <0.01 |
| Body surface area (m2) | 1.9 ± 0.3 | 2.0 ± 0.3 | 2.1 ± 0.3 | 2.2 ± 0.3 | <0.01 |
| 24 hour urine creatinine (mg/d) | 991 ± 415 | 1163 ± 410 | 1412 ± 505 | 1756 ± 645 | <0.01 |
| eGFR (ml/min/1.73m2) | 41 ± 14 | 43 ± 15 | 43 ± 14 | 49 ± 15 | <0.01 |
| Urine albumin (mg/d)† | 61 (11, 376) | 70 (9, 607) | 74 (12, 507) | 66 (10, 615) | 0.63 |
| Serum bicarbonate (mEq/l) | 24.7 ± 3.3 | 24.3 ± 3.3 | 24.2 ± 3.3 | 24.3 ± 3.4 | 0.34 |
| Serum anion gap (mEq/l) | 10.1 ± 2.8 | 10.2 ± 3.1 | 10.1 ± 3.1 | 10.3 ± 3.2 | 0.82 |
| Serum potassium (meq/l) | 4.4 ± 0.5 | 4.3 ± 0.5 | 4.4 ± 0.6 | 4.2 ± 0.5 | <0.01 |
| Dietary | |||||
| Total energy intake (kcal/day)† | 1515 (1116, 2153) | 1512 (1142, 2217) | 1701 (1230, 2256) | 1815 (1379, 2409) | <0.01 |
| Total protein intake (g/day)† | 54 (37, 79) | 59 (42, 78) | 63 (49, 87) | 72 (55, 103) | <0.001 |
| % Protein intake from plant sources | 36% (28%, 45%) | 33% (27%, 40%) | 34% (25%, 42%) | 30% (23%, 37%) | <0.001 |
| Calculated protein intake [Maroni (g/day)]‡ | 46 (35, 56) | 55 (44, 67) | 66 (53, 78) | 82 (66, 101) | <0.001 |
| Ounces of lean meat/fish/poultry (per 2000 kcal) † | 3.4 (2.4, 5.0) | 3.9 (2.7, 5.2) | 4.2 (3.1, 5.7) | 4.7 (3.6, 6.3) | <0.01 |
| Servings of fruits and vegetables (per 2000 kcal)† | 8.0 (4.9, 10.4) | 7.5 (5.2, 10.4) | 7.0 (4.4, 9.4) | 6.5 (4.6, 8.8) | <0.01 |
| Net endogenous acid production- Frasetto equation (mEq/d)ǁ | 37.6 ± 14.0 | 38.0 ± 15.7 | 41.8 ± 15.4 | 46.1 ± 15.0 | <0.01 |
p-value from ANOVA,kruskal-wallis or Chi-squared test, as appropriate.
median (25th, 75th percentile)
Protein intake is calculated from 24 hour urea nitrogen excretion using the Maroni equation
Net endogenous acid production= -10.2 + 54.5*[protein intake (g/d)/potassium intake (mEq/d)]20
Although not associated in univariate models, in multivariable models that account for demographics, diabetes and eGFR, higher net acid excretion was associated with lower serum bicarbonate (0.17 mEq/L lower serum bicarbonate per 10 mEq/day higher net acid excretion; p<0.01), consistent with a larger acid load. Relationships were similar in those with and without diabetes (data not shown). This result was similar if additionally adjusted for smoking status or if removing individuals with a current or former history of smoking (data not shown).
Acid Excretion and Outcomes
We used Cox proportional hazards models to quantify the association between net acid excretion and risk of CKD progression defined as a 50% reduction in eGFR or new-onset end-stage renal disease (ESRD). Sixty-four participants could not be evaluated for this outcome due to lack of follow-up creatinine measurements. Out of these 916 participants, 296 experienced CKD progression over a median follow-up of 5.9 years (25th to 75th percentile: 3.0 to 7.7 years). Contrary to our initial hypothesis, higher quartiles of acid excretion were associated with lower risk of progression in a graded manner in unadjusted and adjusted models (Table 2). Expected acid production may relate to body size with the widely cited expectation of 1 mEq/kg of ideal body weight per day.26 The observed association partially attenuated with adjustment for 24-hour urine creatinine and body mass index in our primary modeling strategy (Table 2).
Table 2. Hazard ratio (95% confidence interval) for net acid excretion and risk of ESRD or 50% decline in eGFR over follow up (296 events) in the full population (n=916).
| Quartile of Net acid excretion | Unadjusted† | +Demographic | + DM/CVD | +eGFR/albuminuria | + ucreatinine/BMI |
|---|---|---|---|---|---|
| Q1 (<20.4mEq/d) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 (20.4–30.7 mEq/d) | 0.95 (0.69, 1.30) | 0.93 (0.68, 1.27) | 1.02 (0.74, 1.39) | 0.94 (0.69, 1.29) | 0.97 (0.71, 1.34) |
| Q3 (30.8–41.8mEq/d) | 0.86 (0.63, 1.18) | 0.89 (0.65, 1.22) | 0.88 (0.64, 1.20) | 0.75 (0.55, 1.04) | 0.83 (0.60, 1.16) |
| Q4 (≥41.9mEq/d) | 0.58(0.42, 0.81) | 0.58 (0.41, 0.82) | 0.55 (0.39, 0.77) | 0.62 (0.44, 0.89) | 0.75 (0.51, 1.11) |
| Continuous per 10 mEq/d higher | 0.88 (0.82, 0.94) | 0.87 (0.81, 0.94) | 0.86 (0.80, 0.92) | 0.88 (0.82, 0.95) | 0.92 (0.84, 1.00) |
| P* | <0.01 | <0.01 | <0.01 | <0.01 | 0.06 |
p is for linear trend derived from a continuous linear model of net acid excretion and outcome
Models are subsequently adjusted for demographics (age, sex, race/ethnicity); followed by history of diabetes mellitus (DM) and cardiovascular disease (CVD); followed by eGFR and albuminuria (log 24-hour urine albumin); followed by 24 hour urine creatinine (ucreatinine) and body mass index (BMI)
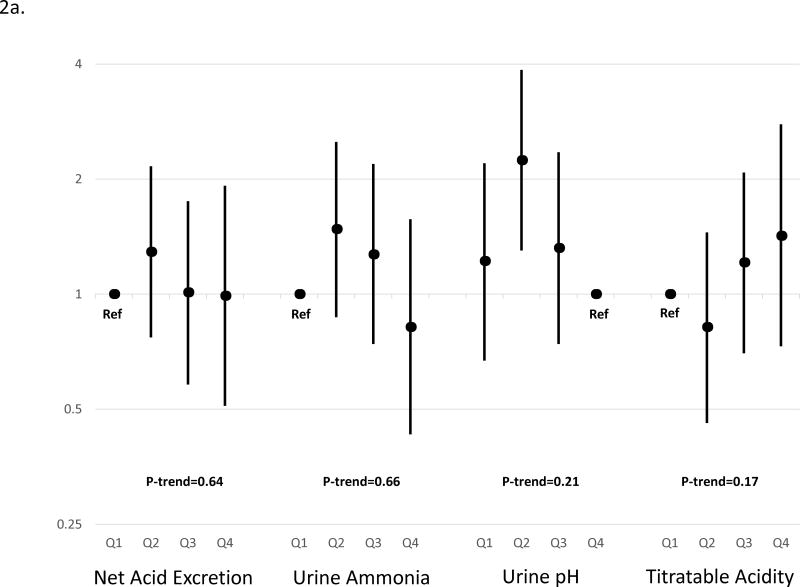
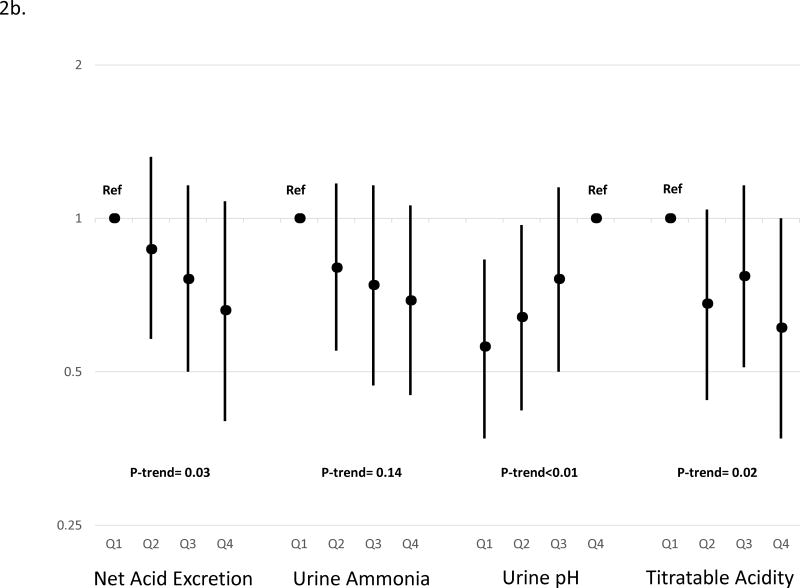
We performed pre-specified analyses stratified by diabetes, due to the impact of diabetes on acid-base physiology.21 In stratified models, the relationship between acid excretion and outcomes differed in patients with diabetes compared to those without (p value for interaction=0.06). Based on the continuous linear model, higher acid excretion was strongly associated with lower risk of CKD progression only among those with diabetes (Table 3). Although the point estimates across quartiles are consistent with a graded effect, the test of the fourth quartile compared to the first quartile was not statistically significant, likely related to reduced power when compared with continuous analyses. When we examined individual components of acid excretion, we found that higher ammonium and titratable acid and lower urine pH each trended toward lower risk of CKD progression with the most robust result for urine pH (p-value for trend<0.01 and p value for interaction for association in those with diabetes vs. without diabetes=0.02; Figure 2; Supplemental Table 1).
Table 3. Hazard ratio (95% confidence interval) for acid-base parameters and risk of ESRD or 50% decline in eGFR over follow up stratified by diabetes mellitus.
| Quartile of Net Acid Excretion | Unadjusted† | +Demographic | + CVD | +eGFR/albuminuria | + ucreatinine/BMI |
|---|---|---|---|---|---|
| Participants without Diabetes (n=459; 107 events) | |||||
| Q1 (<20.4 mEq/d) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 (20.4–30.7 mEq/d) | 1.03 (0.62, 1.70) | 0.93 (0.56, 1.55) | 0.95 (0.57, 1.57) | 1.24 (0.74, 2.07) | 1.29 (0.77, 2.16) |
| Q3 (30.8–41.8 mEq/d) | 0.84 (0.50, 1.43) | 0.83 (0.49, 1.43) | 0.85 (0.50, 1.46) | 0.93 (0.54, 1.59) | 1.01 (0.58, 1.75) |
| Q4 (≥41.9 mEq/d) | 0.64 (0.36, 1.13) | 0.58 (0.33, 1.04) | 0.58 (0.32, 1.03) | 0.84 (0.46, 1.52) | 0.99 (0.51, 1.92) |
| Continuous per 10 mEq/d higher | 0.90 (0.79, 1.02) | 0.88 (0.78, 1.00) | 0.88 (0.78, 1.00) | 0.98 (0.85, 1.12) | 1.04 (0.89, 1.22) |
| P* | 0.11 | 0.06 | 0.05 | 0.74 | 0.64 |
| Participants with Diabetes (n=457; 189 events) | |||||
| Q1 (<20.4 mEq/d) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 (20.4–30.7 mEq/d) | 0.96 (0.64, 1.44) | 1.00 (0.67, 1.49) | 1.05 (0.70, 1.58) | 0.81 (0.54, 1.22) | 0.89 (0.57, 1.33) |
| Q3 (30.8–41.8 mEq/d) | 0.81 (0.55, 1.18) | 0.83 (0.57, 1.23) | 0.87(0.59, 1.28) | 0.64 (0.43, 0.96) | 0.76 (0.50, 1.16) |
| Q4 (≥41.9 mEq/d) | 0.50 (0.33, 0.75) | 0.51 (0.33, 0.78) | 0.52 (0.34, 0.81) | 0.51 (0.33, 0.80) | 0.66 (0.40, 1.08) |
| Continuous per 10 mEq/d higher | 0.84 (0.78, 0.92) | 0.84 (0.77, 0.92) | 0.85 (0.78, 0.93) | 0.84 (0.76, 0.93) | 0.88 (0.80, 0.98) |
| P* | <0.01 | <0.01 | <0.01 | <0.01 | 0.03 |
p is for linear trend derived from a continuous linear model of net acid excretion and outcome
Models are sequentially adjusted for demographics (age, sex, race/ethnicity); followed by history of cardiovascular disease (CVD); followed by eGFR and albuminuria (log 24-hour urine albumin); followed by 24 hour urine creatinine (ucreatinine) and body mass index (BMI)
Figure 2. Relative hazard of End-Stage Renal Disease or 50% decline in estimated glomerular filtration rate by metrics of acid excretion in participants A) without diabetes, and B) with diabetes.
Hazard ratios are adjusted for age, sex, race, cardiovascular disease, estimated glomerular filtration rate, log (24-hour urine albumin), 24-hour urine creatinine, body mass index and stratified by diabetes. Point estimates are indicated by black circles and 95% confidence interval are indicated by error bars. P-values are for linear trend derived from a continuous linear model of acidification parameter and outcome. The p-value for interaction between net acid excretion (continuous model) and diabetes was 0.06. P-values are for linear trend derived from a continuous linear model of net acid excretion and outcome. The p-value for interaction between urine pH (continuous model) and diabetes was 0.02; between urine ammonium (continuous model) and diabetes was 0.38; and between urine titratable acidity (continuous model) and diabetes was 0.01.
Our primary results were similar after additional adjustment for serum bicarbonate (HR 0.88 for each 10 mEq/d higher net acid excretion in participants with diabetes; p=0.02) or serum potassium (data not shown). To prevent residual confounding by body size, we also evaluated models in which net acid excretion was indexed to body surface area or ideal body weight. The results were qualitatively similar (Supplemental Table 2).
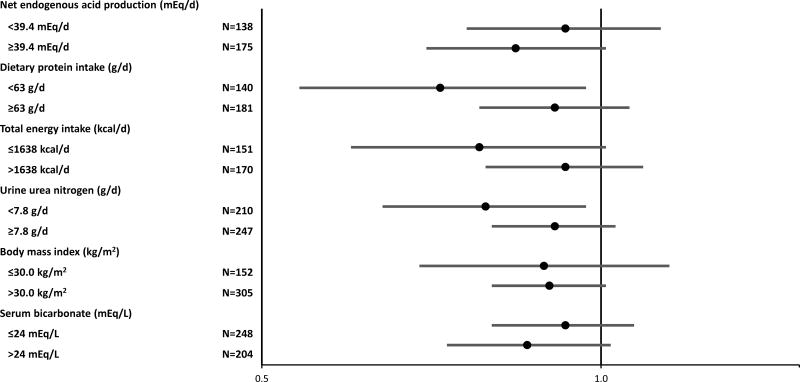
To ensure that our finding of higher risk of CKD progression associated with lower acid excretion was not driven by anorexia or inability to respond appropriately to metabolic acidosis, we evaluated our results stratified by estimated nonvolatile acid load from dietary data, 24-hour urine urea nitrogen, dietary protein intake, total caloric intake, body mass index and serum bicarbonate. Power was limited due to the highly stratified nature of these models; nonetheless, we found similar risk estimates within each stratum (Figure 3).
Figure 3. Relative hazard of End-Stage Renal Disease or 50% decline in estimated glomerular filtration rate per 10 mEq/d higher net acid excretion among diabetic participants and within strata.
Hazard ratios are adjusted for age, sex, race, cardiovascular disease, estimated glomerular filtration rate, log (24-hour urine albumin), 24-hour urine creatinine, body mass index and stratified by diabetes. Point estimates are indicated by black circles and 95% confidence interval are indicated by error bars.
We performed several sensitivity analyses to determine the robustness of our results. We found similar point estimates when modeling risk of ESRD alone or risk of ESRD or death (Supplemental Table 3); when excluding participants who used alkali supplementation or those with urine pH>7.0; when adjusting analyses for UACR in lieu of 24 hour urine albumin (data not shown) or when including urine bicarbonate in the calculation of net acid excretion (Supplemental Table 4). To ensure that a paradoxical effect on survival did not influence our results, we confirmed that net acid excretion did not associate with ESRD-censored death or death alone (Supplemental Table 3).
Estimated Nonvolatile Acid Load and Outcomes
Prior studies have reported higher risk of CKD progression associated with greater acid load as estimated from dietary data.7,8 To determine if inferences are consistent when using directly measured acid excretion versus dietary intake-based estimates of nonvolatile acid load, we evaluated the net endogenous acid production as an “intake-based” estimate of nonvolatile acid load. We calculated net endogenous acid production using intake reported in a food frequency questionnaire and, alternatively, using urinary biomarkers of diet (i.e. urine urea nitrogen and potassium).15
Higher net endogenous acid production measured by food frequency questionnaire (NEAPFFQ) demonstrated the expected associations with greater servings of meat/fish/poultry and lower fruits and vegetables, but also was associated with greater caloric intake overall (Supplemental Table 5). Higher net endogenous acid production estimated from urine biomarkers (NEAPUrine) was more modestly associated with lower fruit and vegetable intake and higher meat/fish/poultry intake, but was independent of total caloric intake (Supplemental Table 6).
Overall, nonvolatile acid load estimated directly from dietary intake as NEAPFFQ was not associated with CKD progression. NEAPUrine was associated with higher risk of CKD progression among those without diabetes (p=0.02 from continuous linear model; Table 4), similar to prior findings in patients with hypertensive CKD.5,7 Higher NEAPUrine was not associated with CKD progression in patients with diabetes (p=0.88 from continuous linear model), and there was evidence of interaction (p value for interaction=0.05).
Table 4. Hazard ratio (95% confidence interval) of ESRD or 50% decline in estimated glomerular filtration rate (eGFR) by quartiles of estimated acid load and stratified by diabetes mellitus.
| No Diabetes | Diabetes | |||
|---|---|---|---|---|
| Unadjusted | Adjusted† | Unadjusted | Adjusted† | |
| NEAPFFQ‡ | ||||
| Q1 (<30.7mEq/d) | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 (30.7–39.3 mEq/d) | 0.84 (0.61, 1.16) | 0.78 (0.56, 1.09) | 0.86 (0.67, 1.11) | 0.83 (0.64, 1.08) |
| Q3 (39.4–50.3 mEq/d) | 1.05 (0.76, 1.44) | 0.76 (0.55, 1.05) | 0.95 (0.74, 1.21) | 1.00 (0.78, 1.29) |
| Q4 (≥50.4 mEq/d) | 1.17 (0.86, 1.60) | 0.89 (0.64, 1.23) | 0.89 (0.69, 1.13) | 0.85 (0.65, 1.10) |
| Continuous per 10 mEq/d higher | 1.03 (0.96, 1.11) | 0.98 (0.90, 1.06) | 1.00 (0.94, 1.05) | 0.99 (0.94, 1.05) |
| P* | 0.36 | 0.61 | 0.88 | 0.82 |
| NEAPUrine‖ | ||||
| Q1 (≤42.2mEq/d) | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 (42.3–57.9 mEq/d) | 1.45 (1.06, 1.99) | 1.16 (0.84, 1.59) | 0.90 (0.74, 1.11) | 1.01 (0.82, 1.25) |
| Q3 (58.0–76.8 mEq/d) | 1.32 (0.96, 1.81) | 1.03 (0.74, 1.42) | 0.67 (0.54, 0.83) | 0.85 (0.68, 1.06) |
| Q4 (≥76.9 mEq/d) | 1.68 (1.24, 2.29) | 1.18 (0.86, 1.61) | 0.89 (0.73, 1.09) | 0.96 (0.78, 1.18) |
| Continuous per 10 mEq/d higher | 1.04 (1.02, 1.06) | 1.03 (1.00, 1.06) | 0.99 (0.96, 1.01) | 1.00 (0.97, 1.02) |
| P* | <0.01 | 0.02 | 0.31 | 0.88 |
p is for linear trend from a continuous model
Models are adjusted for demographics (age, sex, race/ethnicity), history of cardiovascular disease, eGFR, albuminuria (log 24-hour urine albumin), 24-hour urine creatinine and body mass index
NEAPFFQ, Net endogenous acid production from dietary questionnaire; n=2,676 individuals with 813 events due to some individuals missing follow up serum creatinine values for evaluation of 50% reduction in eGFR
NEAPUrine, Net endogenous acid production from urine biomarkers; n=3,175 individuals with 1,039 events due to some individuals missing follow up serum creatinine values for evaluation of 50% reduction in eGFR
Discussion
In this study of acid excretion, acid load, and CKD outcomes, we report that lower 24-hour net acid excretion is associated with higher risk of CKD progression in patients with diabetes. At face value, this finding may warn against efforts to lower acid load with alkali supplements in this population. However, “intake-based” estimates of acid load derived from dietary data were not associated with CKD progression in the patients with diabetes in this study. These otherwise conflicting findings may suggest that the 24-hour net acid excretion result is not primarily a reflection of diet in CKD. The reduction in urine citrate that we observed at lower eGFR suggests that modulation of the diet-independent components of acid load, such as the organic anion production rate, occurs in CKD. Bolstering this view, other groups have recently reported disagreement between estimated and measured acid load in patients with type 2 diabetes mellitus, which they similarly attribute to a potentially altered rate of organic anion production.27 Based on our current findings, we propose a novel hypothesis that a lower rate of diet-independent acid production in diabetes may be associated with risk of disease progression. This hypothesis could also potentially explain a lack of association between serum bicarbonate levels and outcomes in patients with diabetes as noted in other studies.28,29
There are several possible clinical scenarios that should be considered when interpreting our findings about low acid excretion and risk of CKD progression. First, reduced acid excretion could represent low overall food intake or altered body composition, which has been associated with increased risk of CKD progression.30 This does not fully explain our results, because we found similar relationships between those with high and low caloric intake, protein intake, body mass index, and urinary urea nitrogen. Second, lower acid excretion coupled with stable estimates of nonvolatile acid load across the eGFR spectrum may represent net acid retention that could be harmful. This scenario is also unlikely to explain our results because serum bicarbonate was actually higher, not lower, among those with lower acid excretion. A combination of high or preserved serum bicarbonate and lower acid excretion is more likely to represent a lower level of endogenous acid production as opposed to frank acid retention. Thus, we reason that low acid excretion could represent a low rate of diet-independent acid production, including a lower urinary excretion of organic anions, which has been previously shown in patients with diabetes complicated by CKD.31 Under this paradigm, low acid excretion could be a marker of a low rate of organic anion generation in energy producing reactions such as the tricarboxylic acid cycle, or may reflect increased tubular uptake and metabolism of organic anions as a compensation to normalize acid-base status in the setting of reduced tubular function (Figure 4). In animal models, filtered organic anions, such as citrate, are reabsorbed in the proximal tubule in response to acidosis and largely converted to glucose.32-35 Increased tubular citrate uptake could also contribute to lipogenesis by conversion to acetyl-coA via ATP citrate lyase, an enzyme induced in the renal cortex in response to metabolic acidosis.36,37 It is possible that by augmenting renal gluconeogenesis or by diminishing fatty acid oxidation in the proximal tubule this otherwise adaptive response could worsen diabetic kidney disease.38 Alternatively, the presence of these adaptations may be a marker of more advanced tubular disease. This could potentially lead to stronger findings in diabetic patients if hyperfiltration is otherwise masking the severity of underlying CKD.
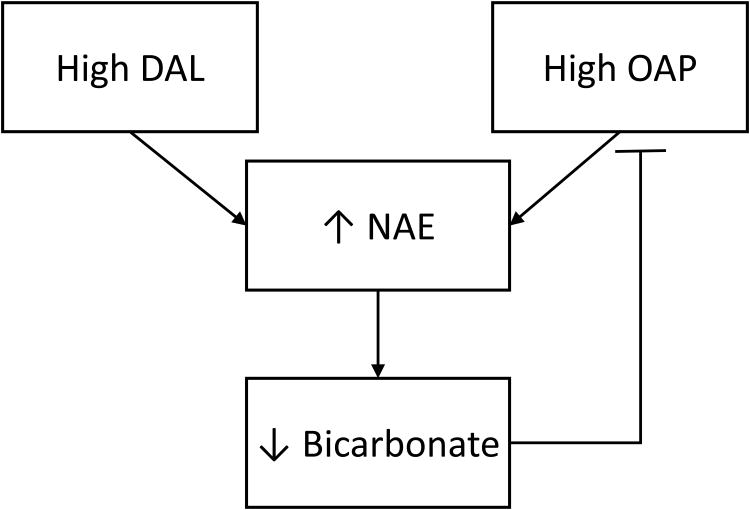
Figure 4. Proposed relationship between diet-dependent acid load (DAL), net acid excretion (NAE), organic anion production rate (OAP) and systemic acid base status.
High diet (DAL) and diet-independent (OAP) components of acid load impact NAE and affect steady-state serum bicarbonate concentration. Worsened systemic acid-base status may feedback to inhibit diet-independent components of acid load, contributing to falling NAE in CKD. Adverse associations between low NAE and outcomes may related to diet-independent components of acid load which reflect greater metabolic compensation for acid-base derangements or impaired tubular functions in CKD.
Our results are consistent with a recent report from the Nephro Test cohort23 in which lower urinary ammonia was associated with higher risk of CKD progression, but those results were not stratified by diabetes status. Interestingly, in our study high urine pH was more strongly associated with CKD progression in patients with diabetes than was low urine ammonium. We do not believe this result is due to inclusion of urine collections with bacterial overgrowth because the reduced risk appeared graded across the spectrum of pH and was robust in sensitivity analyses excluding urines with a more conservative pH cutoff of >7.0. While this could be a chance finding, low urine pH is expected in patients with diabetes to compensate for reduced ammoniagenesis typical of diabetic physiology.10 Patients with diabetes who are unable to lower urine pH may have to invoke greater metabolic compensations to maintain acid-base status. Alternatively, high urine pH could be directly harmful. Nonetheless, urine pH is a simple measurement for further study as a risk marker or treatment target in diabetic CKD.
In our study, diet-based estimates of nonvolatile acid load were not associated with higher risk of CKD progression overall. However, consistent with prior studies in patients with hypertensive kidney disease,5,7 higher NEAP estimated using urine biomarkers of diet (i.e. urine urea nitrogen and potassium)was associated with greater risk of CKD progression in patients without diabetes. This result may reflect improved accuracy of urine-based versus questionnaire-based estimates of acid load, greater independence of these estimates from total energy intake, or the larger sample size. However, it is important to note that urine-based biomarkers are not rigorously validated as indicators of intake in CKD patients.
Our study has several limitations. Urinary parameters were measured in samples that were under long-term storage at -80°C. Prior investigations reveal good recovery of acidification parameters when acidic urine is collected without oil and after freezing, although freezing in these studies was short-term.39,40 Furthermore, in our own quality control samples, we noted high concordance between urine pH before and after a freeze-thaw cycle. We also noticed minimal decreases in urine sulfate and citrate in urines stored at -80°C compared to quality control samples that had been stored at -20°C. As a result, exact values may not be precisely calibrated after our freezing method, however, discrimination of participants with higher versus lower levels was maintained. Titratable acidity was calculated in our study with the assumptions of a negligible contribution of non-phosphate and non-creatinine buffers in the urine, filtration of urine at a pH of 7.4 and a constant pKa for these buffers across urines of different ionic strength.41 Although some of these assumptions may theoretically be inexact, similar methods have been used to measure titratable acidity in other landmark studies of acid base physiology.26 Furthermore, our inferences were similar in analyses of urine ammonium and urine pH alone, each of which were directly measured and thus did not depend upon the accuracy of our titratable acidity calculation. Supporting the validity of our measurements, our detailed physiologic investigations largely recapitulate the expected physiology described in prior studies.10,31,42 Another potential limitation is that our estimates of nonvolatile acid load were generated from food frequency questionnaire results which may not accurately reflect intake. It is possible that low acid excretion could merely reflect differences in diet that were not captured by questionnaire. However, analyses of more objective urinary biomarkers of diet, such as NEAPUrine, were consistent with our conclusions. Finally, the implications of acid excretion should ideally be determined within the context of the systemic acid-base status. We did not have direct measurements of blood pH available to precisely determine acid-base status. Additionally, our sample size was too limited with too few individuals with low serum bicarbonate, who are likely to have metabolic acidosis, to precisely evaluate relationships within bicarbonate strata. Thus, our study may be limited due to errors in measurement of urine net acid excretion in samples not initially collected for this purpose, inaccurate reporting by food frequency questionnaires, and reliance on serum bicarbonate as a metric of systemic acid-base status. Nonetheless, our study accounts for multiple indicators of acid-base homeostasis in CKD including diet, net acid excretion, objective markers of acid production and serum bicarbonate and is among the most comprehensive prospective studies in the field to date.
In sum, our results suggest that alterations in acid-base physiology are strongly associated with risk of CKD progression in those with diabetes. Whether these associations reflect underlying metabolic dysfunction in diabetes or a renal tubular response aimed at maintaining acid base homeostasis requires further study.
Methods
Study Population
The CRIC Study is a multicenter, longitudinal cohort of 3,939 patients with early to moderate CKD who enrolled between 2003–2008 at 13 centers across the United States. All participants had an eGFR between 20–70 ml/min/1.73m2 at study entry, and approximately half had diabetes. Any underlying etiology of kidney disease was included with the exception of polycystic kidney disease, multiple myeloma and glomerulonephritis requiring immunosuppression, however participants did not undergo renal biopsy to determine cause of kidney disease. Detailed inclusion and exclusion criteria have been reported previously.43
Exposure Measurements and Data Collection
We randomly selected 1000 participants with adequate 24-hour urine collections from the baseline visit to measure net acid excretion. Urines were collected without a preservative, immediately mixed, aliquoted and frozen at the clinical sites. Urine samples were stored at -80°C or -20°C prior to testing. Net acid excretion and markers of acid production were measured at Litholink Corporation between 2012–2014 in samples that had been stored at -80°C since collection. Urine pH was measured by pH electrode (n=1000); urine bicarbonate by an enzymatic method using phosphoenol pyruvate (n=1000); urine ammonium by reaction with alpha ketoglutarate (n=1000); urine sulfate by turbidometric assay using barium chloride (n=648) and urine citrate by enzymatic method using citrate lyase (n=648). Measurements below the limit of detection were classified as the lower limit for all analytes. Bicarbonate was not included in the primary measure of net acid excretion because it was below the limit of detection in 93% of tested samples. Nineteen urines with pH≥7.4 were excluded from further analysis due to the concern for bacterial overgrowth, and one urine with pH<4.0 was considered implausible and therefore pH was classified as missing. Quality control samples provided by the laboratory indicated that urine pH values were highly concordant between samples tested immediately and after a freeze-thaw cycle (Supplemental Figure 2).
Urine phosphorus, creatinine, urea nitrogen, sodium and potassium were previously measured in samples stored at -20°C using standard assays.30,44,45 We performed duplicate testing of key analytes in 30 samples stored at -20 deg;C to ensure comparability across sample storage type. Ammonium and pH revealed comparable results, but we noted higher average values of citrate and sulfate in these samples with more variability across measurements (Supplemental Figure 3). Prior studies demonstrate the ability to recover urinary acidification parameters in acidic urine specimens collected without oil and after freezing.39,40
Titratable acid was calculated from total urine phosphorus, creatinine and urine pH using the Henderson Hasselbalch equation (pH = pKa + log ([A-]/[HA])) and pKa's for phosphate (6.8) and creatinine (4.92).41 Similar to direct titration methods, both urinary buffers were assumed to be filtered at a pH of 7.4. The calculation method that we employed has been used in prior studies in the field,26,46 and may circumvent limitations of direct titration methods which could include inadvertent titration of organic anions and the development of precipitates as the pH is increased.47 Total net acid excretion was measured as the sum of urine ammonium and titratable acid. Urine bicarbonate was considered negligible in our primary calculations, based on both our measurements and the expected concentrations in this pH range from prior literature.48 In sensitivity analyses we calculated net acid excretion including urine bicarbonate (net acid excretion= urine ammonium + titratable acid – urine bicarbonate each in mEq).
We estimated nonvolatile acid load from dietary data in the full CRIC study population (n=2,848) using previously published equations for net endogenous acid production [NEAP= 54.5*(dietary protein (g/d)/dietary potassium (mEq/day)) – 10.2].20 Seventy-three participants using alkali supplements were excluded because these are not accounted for in the diet-based estimates. Self-reported dietary intake data were collected using the National Cancer Institute's Diet History Questionnaire, a food frequency questionnaire targeting the intake of 255 common food items over the past year. Total nutrient intakes are calculated using Diet*Calc software (http://appliedresearch.cancer.gov/DHQ/dietcalc/) and adjudication of protein source as animal versus plant-based.49 We calculated net endogenous acid production using urine urea nitrogen as a biomarker of protein intake and 24 hour urine potassium as a biomarker of potassium intake (n=3,385), as previously described.7,50 Participants using potassium chloride supplements were excluded (n=254) because urinary potassium in this equation is a proxy of naturally occurring organic anion salts of potassium, as opposed to chloride salts. Additionally, we excluded participants on alkali supplements that lower acid load, but are not accounted for by this measure (n=90).
Participants' clinical characteristics were evaluated using questionnaires and standardized clinic-based measurements of blood pressure and body anthropometry. Diabetes was defined at baseline by a fasting blood glucose of ≥126 mg/dl or use of diabetic medications.
Outcomes
The primary outcome for this study was the development of ESRD or 50% reduction in eGFR compared to baseline using the CRIC study eGFR equation.51 ESRD was ascertained through active follow-up and linkage with the United States Renal Data System. Serum creatinine was measured annually to estimate eGFR. Due to discrete measurements of eGFR at annual visits, the time of 50% reduction in eGFR was interpolated from these values assuming a linear decline between visits. Additionally, eGFR at the time of ESRD was assumed to be the median national value at initiation of dialysis (10.9 ml/min/1.73m2) where relevant.51 Participants were censored at the time of death, renal transplantation or loss to follow-up. Secondary analyses included the development of ESRD alone or the occurrence of ESRD or death.
Statistical Analysis
To describe acid-base physiology in the CRIC cohort, levels of acid excretion and markers of acid production were described across categories of eGFR (<30; 30–44; 45–59; and 60–90 ml/min/1.73m2) and diabetes. The proportion of acid excreted as ammonium was calculated for each individual and summarized by group. Urinary citrate, in mEq, is a marker of acids produced from organic anion generation and loss. Milliequivalents of citrate in the urine were calculated using the expected average valence at each participants' urine pH utilizing a pKa for citrate of 6.4. The relationship between acid excretion, acid production and serum bicarbonate was assessed using linear regression models adjusted for age, sex, race, eGFR and diabetes.
The relationship between baseline net acid excretion or estimates of nonvolatile acid load and clinical outcomes were evaluated using Cox proportional hazards models with sequential adjustment for demographics (age, sex, race/ethnicity), comorbidity (diabetes and history of cardiovascular disease), kidney function (eGFR and log 24-hour urine albumin) and body composition (24-hour urine creatinine and body mass index). We performed pre-specified analyses stratified by diabetes and tested the interaction using multiplicative interaction terms with net acid excretion as a continuous variable. The proportionality assumption was assessed using Schoenfeld residuals. Age violated the proportionality assumption, but similar results were obtained for the parameters of interest in models stratified by age, therefore this was not deemed meaningful in these analyses.
Sensitivity Analyses
We performed a series of sensitivity analyses to determine the robustness of our primary findings. Due to concern for residual confounding by body composition we performed analyses in which net acid excretion was indexed to body surface area and ideal body weight. Because total 24-hour urine phosphorus and creatinine contribute to our calculation of titratable acidity and also may associate with outcomes, we evaluated individual components of acid excretion separately including 24-hour urine ammonium, pH and titratable acid. Finally, to ensure that our results were not due to the influence of inadequate nutrition or frankly abnormal acid-base status, we performed stratified analyses across groups defined by serum bicarbonate, body mass index, total energy intake, protein intake, and urine urea nitrogen.
All analyses were performed using Stata SE 13.1 (College Station, Texas, USA).
Supplementary Material
Acknowledgments
We gratefully acknowledge the contributions of CRIC participants and staff and statistical consultation from Huiman Barnhart, PhD. Portions of this study were presented as an oral abstract at the American Society of Nephrology Kidney Week on November 6, 2015 in San Diego, CA.
This study was supported by K23DK095949 (JS), R01DK081374 (MW) and K24DK093723 (MW), and P30DK096493, each from the National Institute of Diabetes and Digestive and Kidney Diseases. Funding for the CRIC study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the University of Pennsylvania CTRC CTSA UL1 RR-024134, Johns Hopkins University UL1 RR-025005, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1RR024986, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, and Kaiser NIH/NCRR UCSF-CTSI UL1 RR-024131. MD was supported by 13FTF15920005.
Footnotes
Disclosures: None
References
- 1.Dobre M, Yang W, Chen J, et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2013;62(4):670–678. doi: 10.1053/j.ajkd.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2010;79(3):356–362. doi: 10.1038/ki.2010.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum Bicarbonate Levels and the Progression of Kidney Disease: A Cohort Study. Am J Kidney Dis. 2009;54(2):270–277. doi: 10.1053/j.ajkd.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate Supplementation Slows Progression of CKD and Improves Nutritional Status. J Am Soc Nephrol. 2009;20(9):2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010;78(3):303–309. doi: 10.1038/ki.2010.129. [DOI] [PubMed] [Google Scholar]
- 6.Scialla JJ. The balance of the evidence on acid-base homeostasis and progression of chronic kidney disease. Kidney Int. 2015;88(1):9–11. doi: 10.1038/ki.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scialla JJ, Appel LJ, Astor BC, et al. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 2012;82(1):106–112. doi: 10.1038/ki.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee T, Crews DC, Wesson DE, et al. High Dietary Acid Load Predicts ESRD among Adults with CKD. J Am Soc Nephrol. 2015;26(7):1693–1700. doi: 10.1681/ASN.2014040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012;81(1):86–93. doi: 10.1038/ki.2011.313. [DOI] [PubMed] [Google Scholar]
- 10.Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K. Urine Composition in Type 2 Diabetes: Predisposition to Uric Acid Nephrolithiasis. J Am Soc Nephrol. 2006;17(5):1422–1428. doi: 10.1681/ASN.2005121246. [DOI] [PubMed] [Google Scholar]
- 11.Chan JC. Urinary organic anions: clinical significance and evaluation of a method for determination and preservation. Clin Biochem. 1972;5(3):182–185. doi: 10.1016/s0009-9120(72)80029-8. [DOI] [PubMed] [Google Scholar]
- 12.Lemann J, Jr, Relman AS. The relation of sulfur metabolism to acid-base balance and electrolyte excretion: the effects of DL-methionine in normal man. J Clin Invest. 1959;38:2215–2223. doi: 10.1172/JCI104001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lennon EJ, Lemann J., Jr Influence of diet composition on endogenous fixed acid production. Am J Clin Nutr. 1968;21(5):451–456. doi: 10.1093/ajcn/21.5.451. [DOI] [PubMed] [Google Scholar]
- 14.Remer T. Influence of diet on acid-base balance. Semin Dial. 2000;13(4):221–226. doi: 10.1046/j.1525-139x.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- 15.Scialla JJ. Dietary Acid Load: A Novel Nutritional Target in Chronic Kidney Disease? Adv Chronic Kidney Dis. 2013;20(2):141–149. doi: 10.1053/j.ackd.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiess W, Ayer J, Lotspeich W, Pitts R. The renal regulation of acid-base balance in man. II. Factors affecting the excretion of titratable acid by the normal human subject. J Clin Invest. 1948;27(1):57–64. doi: 10.1172/JCI101924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koeppen BM. The kidney and acid-base regulation. Adv Physiol Educ. 2009;33(4):275–281. doi: 10.1152/advan.00054.2009. [DOI] [PubMed] [Google Scholar]
- 18.Hood VL, Tannen RL. Protection of acid-base balance by pH regulation of acid production. N Engl J Med. 1998;339(12):819–826. doi: 10.1056/NEJM199809173391207. [DOI] [PubMed] [Google Scholar]
- 19.Packer RK, Curry CA, Brown KM. Urinary organic anion excretion in response to dietary acid and base loading. J Am Soc Nephrol. 1995;5(8):1624–1629. doi: 10.1681/ASN.V581624. [DOI] [PubMed] [Google Scholar]
- 20.Frassetto LA, Lanham-New SA, Macdonald HM, et al. Standardizing terminology for estimating the diet-dependent net acid load to the metabolic system. J Nutr. 2007;137(6):1491–1492. doi: 10.1093/jn/137.6.1491. [DOI] [PubMed] [Google Scholar]
- 21.Moranne O, Froissart M, Rossert J, et al. Timing of Onset of CKD-Related Metabolic Complications. J Am Soc Nephrol. 2009;20(1):164–171. doi: 10.1681/ASN.2008020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman AD, Lemann J, Jr, Lennon EJ, Relman AS. Production, Excretion, and Net Balance of Fixed Acid in Patients with Renal Acidosis. J Clin Invest. 1965;44:495–506. doi: 10.1172/JCI105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallet M, Metzger M, Haymann JP, et al. Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int. 2015;88(1):137–145. doi: 10.1038/ki.2015.52. [DOI] [PubMed] [Google Scholar]
- 24.Wesson DE, Simoni J, Broglio K, Sheather S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol. 2011;300(4):F830–837. doi: 10.1152/ajprenal.00587.2010. [DOI] [PubMed] [Google Scholar]
- 25.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline Characteristics and Associations with Kidney Function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lennon EJ, Lemann J, Jr, Litzow JR. The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest. 1966;45(10):1601–1607. doi: 10.1172/JCI105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frassetto LA, Shi L, Schloetter M, Sebastian A, Remer T. Established dietary estimates of net acid production do not predict measured net acid excretion in patients with Type 2 diabetes on Paleolithic-Hunter-Gatherer-type diets. Eur J Clin Nutr. 2013;67(9):899–903. doi: 10.1038/ejcn.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navaneethan SD, Schold JD, Arrigain S, et al. Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(10):2395–2402. doi: 10.2215/CJN.03730411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schutte E, Lambers Heerspink HJ, Lutgers HL, et al. Serum Bicarbonate and Kidney Disease Progression and Cardiovascular Outcome in Patients With Diabetic Nephropathy: A Post Hoc Analysis of the RENAAL (Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan) Study and IDNT (Irbesartan Diabetic Nephropathy Trial) Am J Kidney Dis. 2015;66(3):450–458. doi: 10.1053/j.ajkd.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Wilson FP, Xie D, Anderson AH, et al. Urinary Creatinine Excretion, Bioelectrical Impedance Analysis, and Clinical Outcomes in Patients with CKD: The CRIC Study. Clin J Am Soc Nephrol. 2014;9(12):2095–2103. doi: 10.2215/CJN.03790414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma K, Karl B, Mathew AV, et al. Metabolomics Reveals Signature of Mitochondrial Dysfunction in Diabetic Kidney Disease. J Am Soc Nephrol. 2013;24(11):1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown JC, Packer RK, Knepper MA. Role of organic anions in renal response to dietary acid and base loads. Am J Physiol. 1989;257(2 Pt 2):F170–176. doi: 10.1152/ajprenal.1989.257.2.F170. [DOI] [PubMed] [Google Scholar]
- 33.Simpson DP, Hager SR. pH and Bicarbonate Effects on Mitochondrial Anion Accumulation: PROPOSED MECHANISM FOR CHANGES IN RENAL METABOLITE LEVELS IN ACUTE ACID-BASE DISTURBANCES. J Clin Invest. 1979;63(4):704–712. doi: 10.1172/JCI109353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins AD, Dousa TP, Smith LH. Transport of citrate across renal brush border membrane: effects of dietary acid and alkali loading. Am J Physiol. 1985;249(4 Pt 2):F590–595. doi: 10.1152/ajprenal.1985.249.4.F590. [DOI] [PubMed] [Google Scholar]
- 35.Simpson D. Citrate excretion: a window on renal metabolism. Am J Physiol. 1983;244(3):223–234. doi: 10.1152/ajprenal.1983.244.3.F223. [DOI] [PubMed] [Google Scholar]
- 36.Melnick JZ, Srere PA, Elshourbagy NA, Moe OW, Preisig PA, Alpern RJ. Adenosine triphosphate citrate lyase mediates hypocitraturia in rats. J Clin Invest. 1996;98(10):2381–2387. doi: 10.1172/JCI119051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chypre M, Zaidi N, Smans K. ATP-citrate lyase: A mini-review. Biochem Biophys Res Comm. 2012;422(1):1–4. doi: 10.1016/j.bbrc.2012.04.144. [DOI] [PubMed] [Google Scholar]
- 38.Kang HM, Ahn SH, Choi P, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21(1):37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi JH, Shin HJ, Kim SM, Han SW, Kim HJ, Oh MS. Does the exposure of urine samples to air affect diagnostic tests for urine acidification. Clin J Am Soc Nephrol. 2012;7:1211–1216. doi: 10.2215/CJN.03230312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remer T, Montenegro-Bethancourt G, Shi L. Long-term urine biobanking: Storage stability of clinical chemical parameters under moderate freezing conditions without use of preservatives. Clin Biochem. 2014;47:307–311. doi: 10.1016/j.clinbiochem.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz WB, Bank N, Cutler RW. The influence of urinary ionic strength on phosphate pK2′ and the determination of titratable acid. J Clin Invest. 1959;38(2):347–356. doi: 10.1172/JCI103808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Metabolic Basis for Low Urine pH in Type 2 Diabetes. Clin J Am Soc Nephrol. 2010;5(7):1277–1281. doi: 10.2215/CJN.08331109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 44.He J, Mills K, Appel LJ, et al. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol. 2016;27(4):1202–12. doi: 10.1681/ASN.2015010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemann J, Jr, Adams ND, Wilz DR, Brenes LG. Acid and mineral balances and bone in familial proximal renal tubular acidosis. Kidney Int. 2000;58(3):1267–1277. doi: 10.1046/j.1523-1755.2000.00282.x. [DOI] [PubMed] [Google Scholar]
- 47.Kok DJ, Poindexter J, Pak CY. Calculation of titratable acidity from urinary stone risk factors. Kidney Int. 1993;44(1):120–126. doi: 10.1038/ki.1993.221. [DOI] [PubMed] [Google Scholar]
- 48.Gamble J. Carbonic Acid and Bicarbonate in Urine. J Biol Chem. 1922;51:295–310. [Google Scholar]
- 49.Scialla JJ, Appel LJ, Wolf M, et al. Plant protein intake is associated with fibroblast growth factor 23 and serum bicarbonate levels in patients with chronic kidney disease: the Chronic Renal Insufficiency Cohort study. J Ren Nutr. 2012;22(4):379–388. e371. doi: 10.1053/j.jrn.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scialla JJ, Appel LJ, Astor BC, et al. Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(7):1526–1532. doi: 10.2215/CJN.00150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang W, Xie D, Anderson AH, et al. Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2014;63(2):236–243. doi: 10.1053/j.ajkd.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kidney Disease: Improving Global Outcomes (KIDGO) CKD Working Group. KDIGO 2012 Clinical Practice Guidelines for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;(3):1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.