Figure 3. ACAT2 is ubiquitinated by K48-specific ubiquitin linkages.
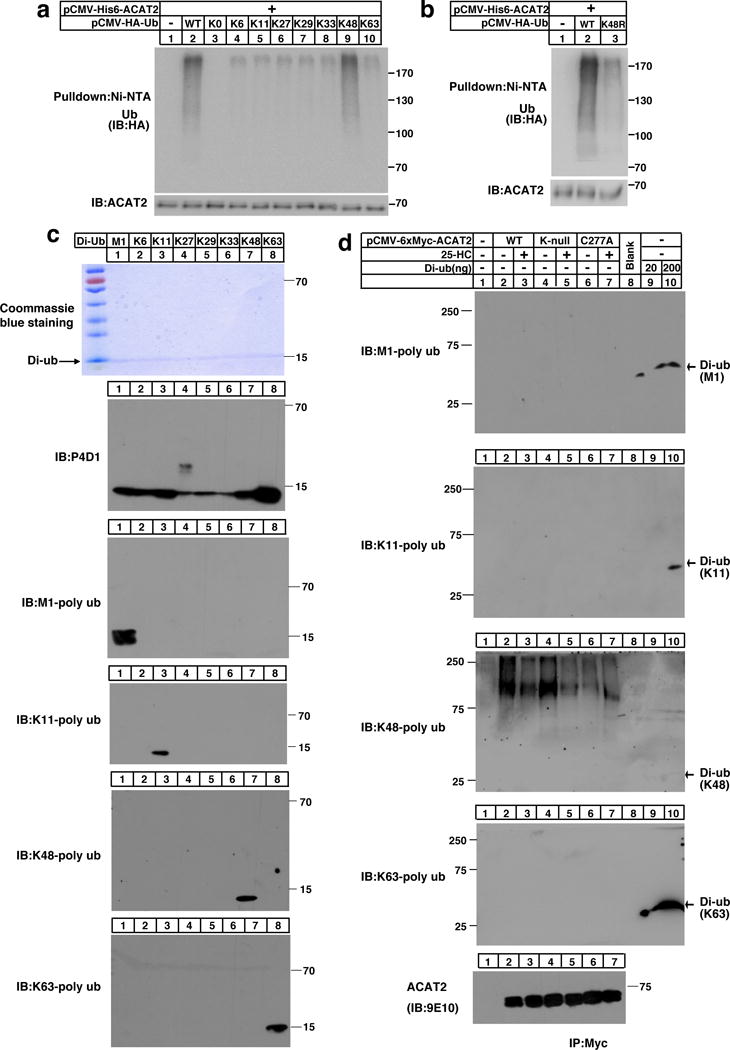
(a–b) CHO cells co-transfected with His6 tagged-ACAT2 and HA tagged-Ub ((WT, K0, K6-only, K11-only, K27-only, K29-only, K33-only, K48-only, or K63-only) (a) or (K48R) (b)) were depleted of lipids for 27 hrs. Cells were then treated with MG132 for another 5 hrs. Cells were harvested and lysed in the denaturing IP buffer, and subsequently subjected to ubiquitination analysis. The immunoblots are representative of 3 independent experiments.
(c) 200 ng of M1-linked (M1), K6-linked (K6), K11-linked (K11), K27-linked (K27), K29-linked (K29), K33-linked (K33), K48-linked (K48) or K63-linked (K63) ubiquitin dimers were separated by SDS-PAGE and probed with different anti-ubiquitin antibodies as indicated. The coommassie brilliant blue-stained gel is shown as a loading control. The independent repeat of these experiments is shown in Supplementary Fig. 3a.
(d) CHO cells were transfected with pCMV-HA-Ub together with the plasmid expressing Myc-tagged ACAT2 (WT), (K-null) or (C277A) and depleted of lipids for 16 hrs. Cells were treated with or without 25-HC for 11 hrs. Then MG132 was added into medium for another 5 hrs. Cells were harvested and lysed. The ACAT2 proteins were immunoprecipitated by anti-Myc antibodies coupled agarose and eluted with Myc peptides. Western blotting was carried out using different ubiquitin linkage antibodies or anti-Myc antibody as indicated. The di-ubiquitin was loaded about 30 min later than ACAT2 samples and served as controls. The independent repeat of these experiments is shown in Supplementary Fig. 3b.
Uncropped blots are shown in Supplementary Fig. 8.
