Figure 6.
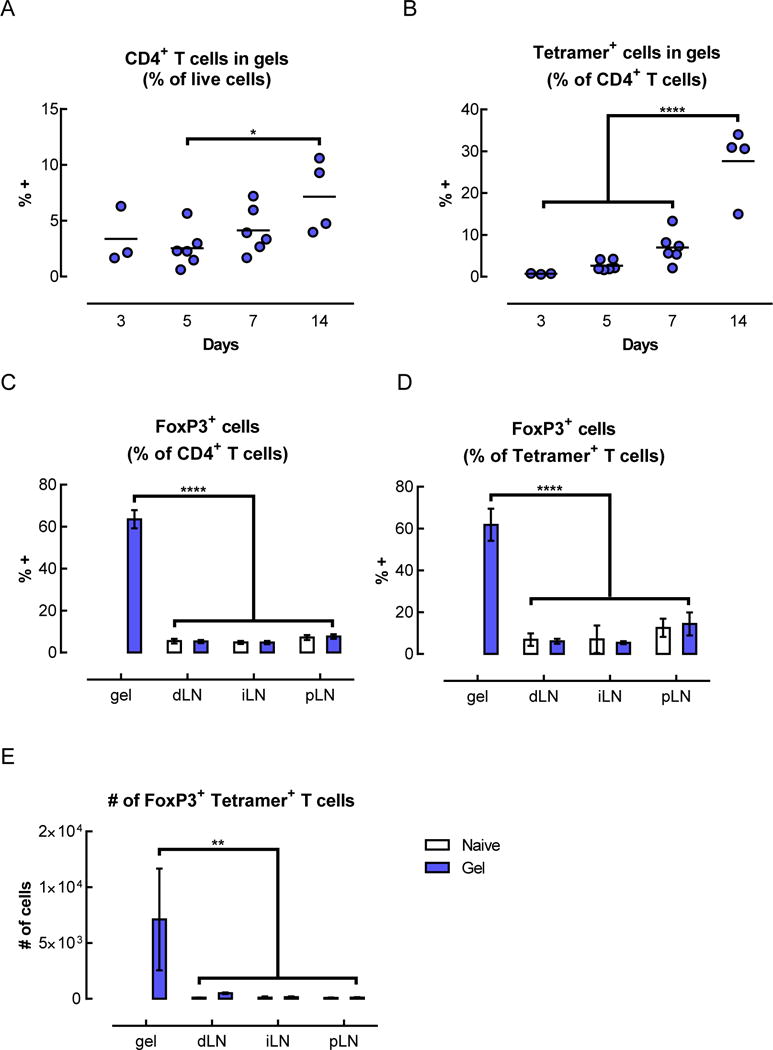
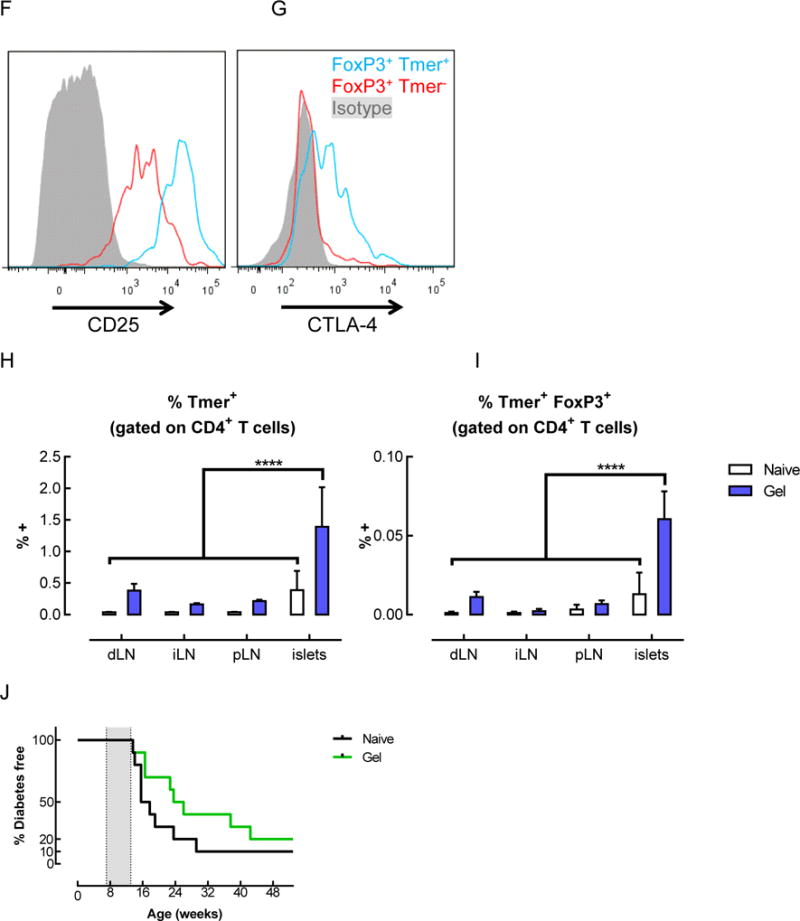
Tetramer+ FoxP3+ CD4+ T cells were enriched in the gels and pancreatic islets of mice that received BDC peptide-conjugated pore-forming gels. (A–G) Flow cytometric analysis of T cells isolated from gels 14 days after subcutaneous injection of gels delivering AuNP/GM-CSF and BDC peptide-conjugated alginate. The percentage of CD4+ cells (A) and tetramer+ CD4+ cells (B) were analyzed. (n = 2 – 6; individual data points and mean shown; * p < 0.05; **** p < 0.0001; all conditions were compared to each other; statistical tests were only performed for the conditions where n >= 3.) (C–E) Analysis of the CD4+ cells in the gels at day 14, showing the fraction of CD4+ cells that were FoxP3+ (C), the fraction of tetramer+ cells that were FoxP3+ (D), and the number of FoxP3+ tetramer+ CD4+ T cells in the gels (E), as calculated by adjusting for total cell number. (n = 3; mean ± s.d. shown; ** p < 0.01; **** p < 0.0001; all conditions were compared to each other.) (F–G) Expression of CD25 (F) and CTLA-4 (G) on tetramer+ CD4+ T cells isolated from peptide-conjugated gels after 14 days. (H–I) Flow cytometric analysis of T cells in the lymph nodes and pancreatic islets of naïve mice or mice that received BDC peptide-conjugated alginate gels. Percentage of tetramer+ cells (H) and tetramer+ FoxP3+ cells (I) among CD4+ T cells at day 14. (J) Spontaneously occurring diabetes study in NOD mice. 3 doses of gels delivering AuNP/GM-CSF and peptide conjugated to alginate were administered between 7–13 weeks (grey shaded region), and mice were monitored for the development of diabetes. (n = 10; p = 0.1259.)
