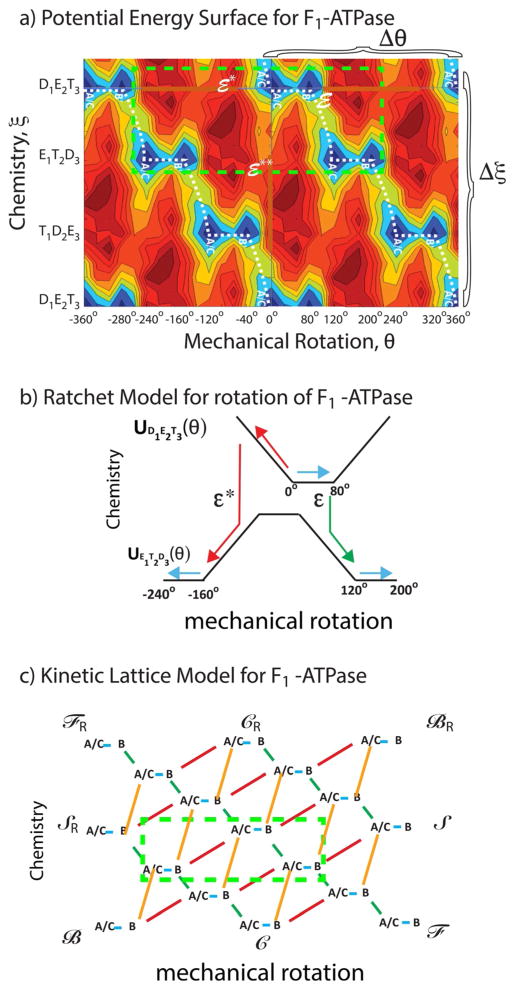
Figure 1.
a) Energy landscape for the F1 ATPase from the work of Warshel and Mukherjee.[30] The fundamental periods Δξ and Δθ for which U(ξ, θ)=U(ξ ± iΔξ, θ ± jΔθ) with i,j = any integers, are shown. b) A “ratchet” representation in terms of two 1D energy profiles with transitions between them for the two chemical states D1E2T3 and E1T2D3 from −240° to +200° (the area enclosed in the bright green dashed box in Figure 1a). The remarkable and salient point is that the mechanism shown by the green arrows (clockwise rotation of 120°) seems by common sense to be far more likely than that shown by the red arrows (counterclockwise rotation by 160°), but, in fact, if ε*=ε these two processes are equally likely, and if ε*<ε counterclockwise rotation (red path) is more likely than clockwise rotation (green path). c) A kinetic lattice model describing the potential energy landscape, where green indicates transition over the barrier (saddle point) ε and red indicates transition over the energy barrier (saddle point) ε*. The dashed box illustrates the part of the kinetic lattice corresponding to the region enclosed in the bright green box in Figure 1a. Four different cycles and their microscopic reverses can be identified, ℱ/ℱR in which clockwise rotation is coupled to ATP hydrolysis, ℬ/ℬR in which counterclockwise rotation is coupled to ATP hydrolysis, 𝒮/𝒮R (slip) in which rotation occurs uncoupled to chemistry, and 𝒞/𝒞R (futile cycling) in which chemistry occurs uncoupled to roation.