Table 1.
The first report of Rh(III)-catalyzed C(sp2)–H annulation with internal alkynes using the N-methoxy amide directing group (Guimond and Fagnou et. al., 2010).[29]
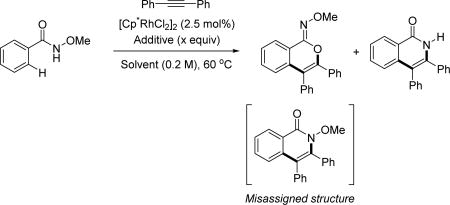
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | Solvent | Additive (x equiv) | % Yield[a] (O+N)-CP[c] |
Ratio (O:N)-CP[c] |
| 1 | DMF | Cu(OAc)2·H2O (2) | 89 | 1:1.1 |
| 2 | DMF | CsOAc (2) | 38 | 1:20 |
| 3 | MeOH | CsOAc (2) | 97 (92)[b] | 1:20 |
| 4 | MeOH | CsOAc (0.3) | 97 (90)[b] | 1:20 |
1H NMR yield.
Isolated yield.
CP = Cyclized Product.
Cp* = pentamethylcyclopentadienyl.
