Abstract
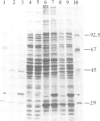
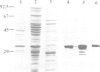
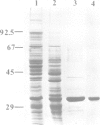
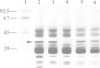
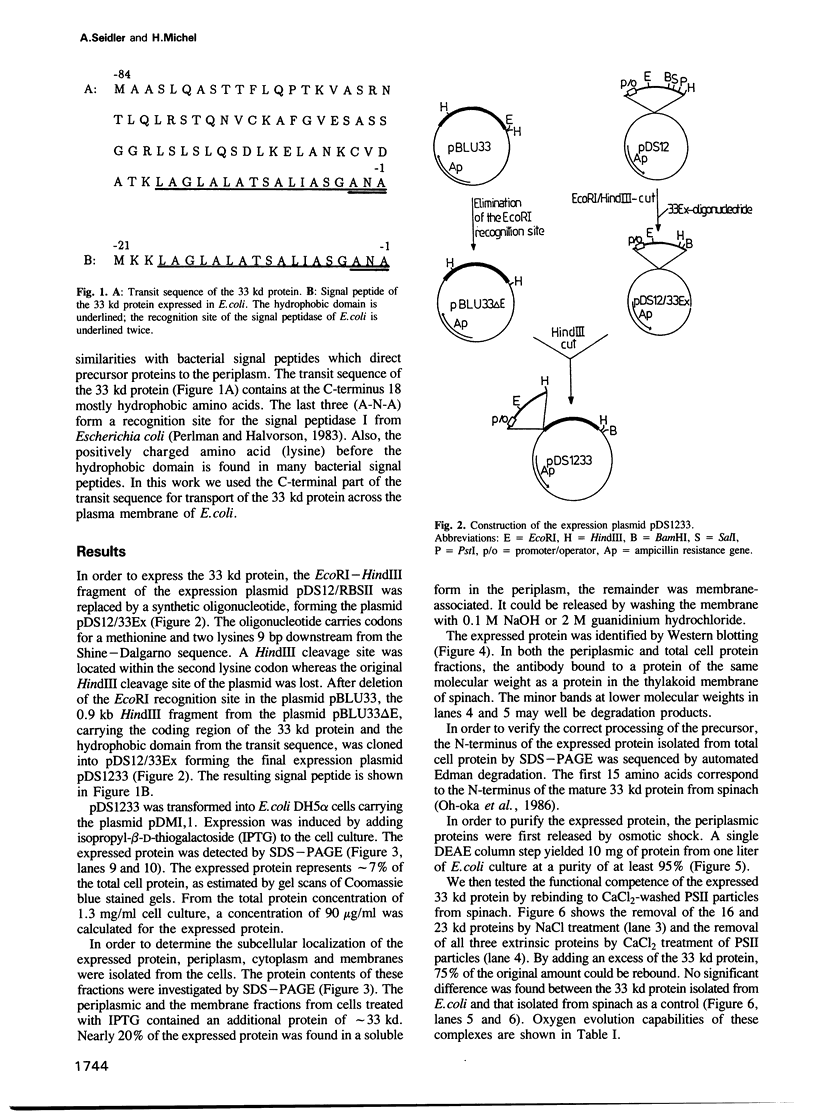
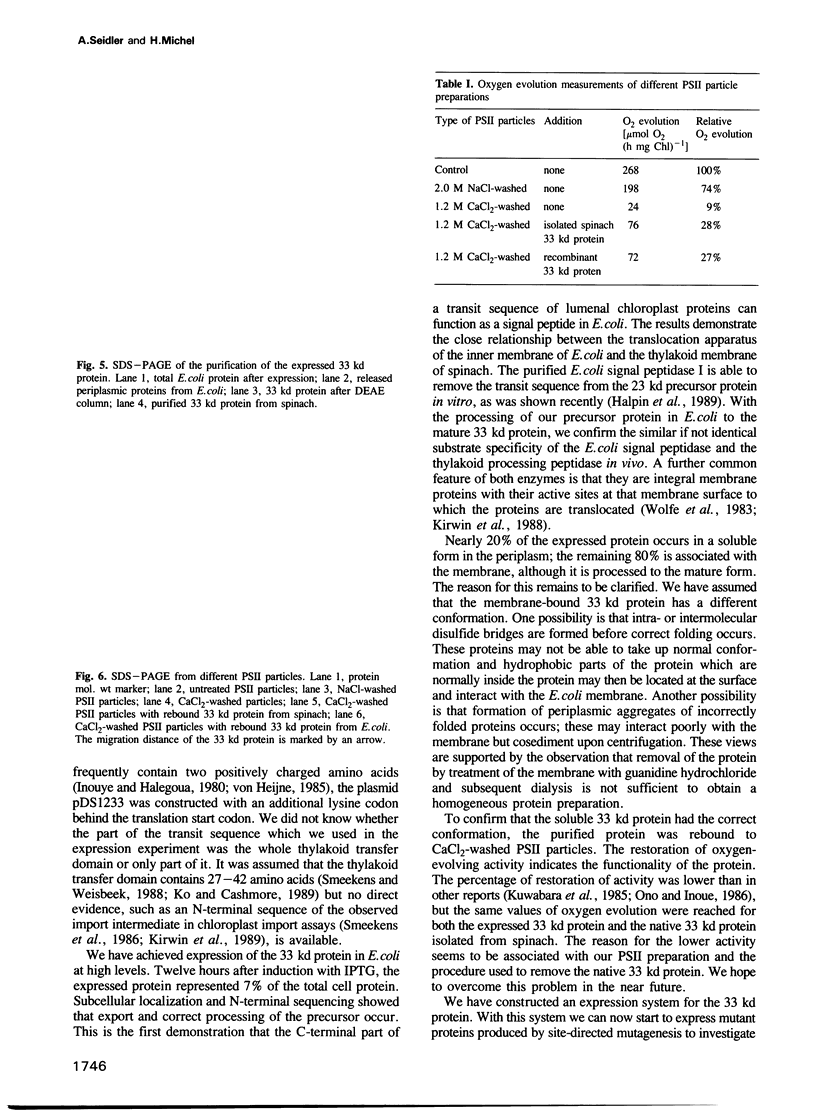
The cDNA for the 33 kd protein from the oxygen-evolving complex of spinach together with the coding region for the hydrophobic C-terminal part of the transit sequence was cloned into the expression plasmid pDS12/33Ex. The 33 kd protein precursor was expressed in Escherichia coli, secreted into the periplasm and correctly processed to the mature 33 kd protein. Thus the hydrophobic domain of the transit sequence, preceded by a methionine and two lysine residues, can function as a bacterial signal peptide. The periplasmic proteins were released from the cells by osmotic shock and the expressed protein was purified by anion exchange chromatography. The protein was identified by SDS-PAGE and Western blotting. N-terminal sequence analysis showed that the cleavage of the signal peptide occurred at the correct position. The expressed protein could be rebound to CaCl2-washed PSII particles and oxygen evolution was restored in equal amounts by the 33 kd protein from both E. coli and spinach.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki S., Murata K., Ushio K., Sakai R. Dose-response relationship between tobacco consumption and melanin pigmentation in the attached gingiva. Arch Environ Health. 1983 Nov-Dec;38(6):375–378. doi: 10.1080/00039896.1983.10545823. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujard H., Gentz R., Lanzer M., Stueber D., Mueller M., Ibrahimi I., Haeuptle M. T., Dobberstein B. A T5 promoter-based transcription-translation system for the analysis of proteins in vitro and in vivo. Methods Enzymol. 1987;155:416–433. doi: 10.1016/0076-6879(87)55028-5. [DOI] [PubMed] [Google Scholar]
- Certa U., Bannwarth W., Stüber D., Gentz R., Lanzer M., Le Grice S., Guillot F., Wendler I., Hunsmann G., Bujard H. Subregions of a conserved part of the HIV gp41 transmembrane protein are differentially recognized by antibodies of infected individuals. EMBO J. 1986 Nov;5(11):3051–3056. doi: 10.1002/j.1460-2075.1986.tb04605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffaud G. D., March P. E., Inouye M. Expression and secretion of foreign proteins in Escherichia coli. Methods Enzymol. 1987;153:492–507. doi: 10.1016/0076-6879(87)53074-9. [DOI] [PubMed] [Google Scholar]
- Halpin C., Elderfield P. D., James H. E., Zimmermann R., Dunbar B., Robinson C. The reaction specificities of the thylakoidal processing peptidase and Escherichia coli leader peptidase are identical. EMBO J. 1989 Dec 1;8(12):3917–3921. doi: 10.1002/j.1460-2075.1989.tb08572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Kirwin P. M., Elderfield P. D., Williams R. S., Robinson C. Transport of proteins into chloroplasts. Organization, orientation, and lateral distribution of the plastocyanin processing peptidase in the thylakoid network. J Biol Chem. 1988 Dec 5;263(34):18128–18132. [PubMed] [Google Scholar]
- Kirwin P. M., Meadows J. W., Shackleton J. B., Musgrove J. E., Elderfield P. D., Mould R., Hay N. A., Robinson C. ATP-dependent import of a lumenal protein by isolated thylakoid vesicles. EMBO J. 1989 Aug;8(8):2251–2255. doi: 10.1002/j.1460-2075.1989.tb08349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K., Cashmore A. R. Targeting of proteins to the thylakoid lumen by the bipartite transit peptide of the 33 kd oxygen-evolving protein. EMBO J. 1989 Nov;8(11):3187–3194. doi: 10.1002/j.1460-2075.1989.tb08477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libby R. T., Braedt G., Kronheim S. R., March C. J., Urdal D. L., Chiaverotti T. A., Tushinski R. J., Mochizuki D. Y., Hopp T. P., Cosman D. Expression and purification of native human granulocyte-macrophage colony-stimulating factor from an Escherichia coli secretion vector. DNA. 1987 Jun;6(3):221–229. doi: 10.1089/dna.1987.6.221. [DOI] [PubMed] [Google Scholar]
- Mei R., Green J. P., Sayre R. T., Frasch W. D. Manganese-binding proteins of the oxygen-evolving complex. Biochemistry. 1989 Jun 27;28(13):5560–5567. doi: 10.1021/bi00439a033. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem. 1984 Jul 16;142(2):337–342. doi: 10.1111/j.1432-1033.1984.tb08291.x. [DOI] [PubMed] [Google Scholar]
- Smeekens S., Bauerle C., Hageman J., Keegstra K., Weisbeek P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 1986 Aug 1;46(3):365–375. doi: 10.1016/0092-8674(86)90657-4. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W., Goodman J. M. Sequence of the leader peptidase gene of Escherichia coli and the orientation of leader peptidase in the bacterial envelope. J Biol Chem. 1983 Oct 10;258(19):12073–12080. [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]