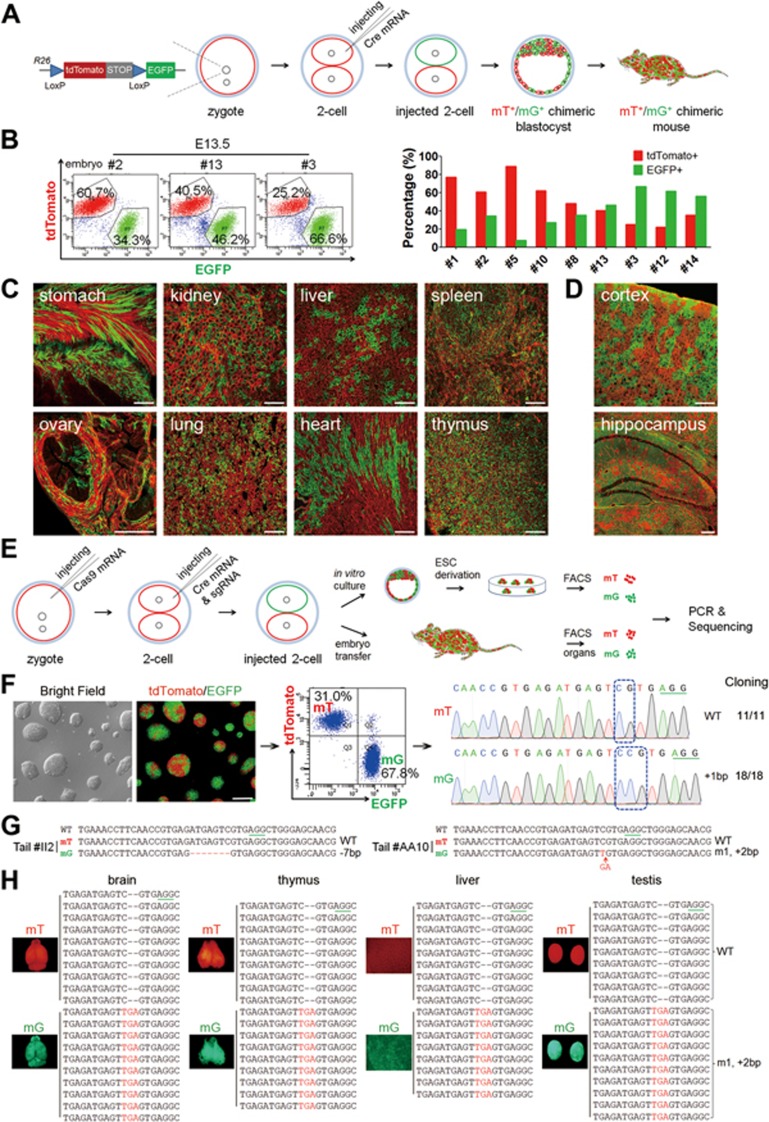
Figure 1.
A two-step injection method (termed 2CC) for rapid mutation of genes of interest in mouse chimeras. (A) Experimental procedure for Cre mRNA injection into one blastomere of mouse two-cell stage embryos to generate tdTomato+/EGFP+ (mT+/mG+) chimeras. (B) FACS analysis and quantification of mT+ and mG+ cells in dissociated whole-embryo suspensions of E13.5 embryos (left); in the quantitation, each red or green bar pair represents one mouse embryo (right). (C) Cryosection of tissues from a newborn mT+/mG+ chimera (P17). All tested tissues showed mT+ and mG+ fluorescence. Scale bar, 100 μm. (D) Cryosection of cortex and hippocampus of an adult chimera (2 months old) showing chimeric distribution of mT+ and mG+ cells. Scale bar, 100 μm. (E) Experimental procedure for the two-step injection method: Cas9 mRNA was injected into the zygotes first, followed by coinjection of Cre mRNA and sgRNA into one blastomere of two-cell stage embryos on the following day. Injected two-cell embryos were cultured in vitro to blastocysts to derive ESC lines. To generate chimeric mice, injected two-cell embryos were transplanted into oviducts of pseudopregnant females. (F) A representative Tet3-targeted ESC line derived from a Tet3-targeted mT+/mG+ blastocyst, and its corresponding FACS and sequencing results. mT+ cells carried WT alleles (11 in 11 sequenced alleles), whereas mG+ cells carried mutant alleles (18 in 18 sequenced alleles). Scale bar, 100 μm. (G) Sequencing results of FACS-sorted mT+ and mG+ cells from Tet3-targeted chimeric mouse tails. Tet3-targeted mG+ cells exhibited only one mutant peak, whereas the mT+ cells from the same mouse showed only WT peak. (H) Whole-mount fluorescence analysis of organs from one Tet3-targeted chimeric mouse (#AA10) and the corresponding sequencing results of mT+ and mG+ cells from these organs. All tested organs exhibited the same genotype as the corresponding tail shown in G.