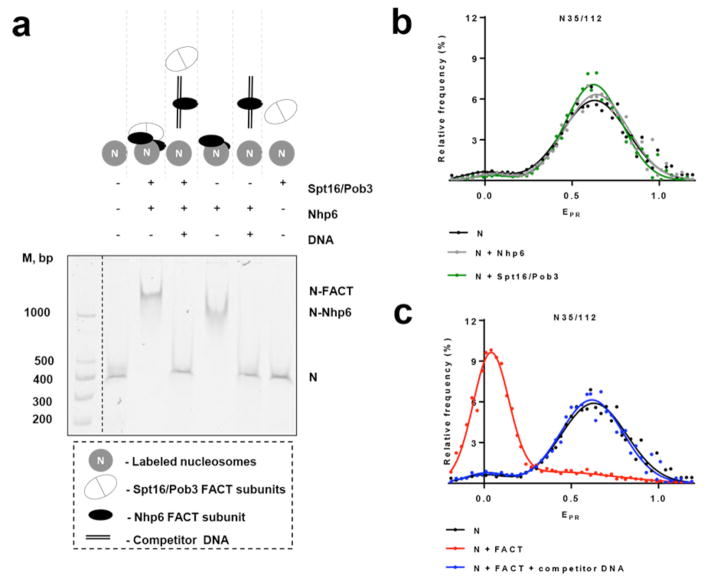
Fig. 2. Nucleosome unfolding by yFACT is extensive and reversible.
a, Binding of FACT and different combinations of its subunits to fluorescently labeled N35/112 nucleosomes analyzed by native PAGE. Fluorescence of Cy5 (nucleosomal templates) or FAM (DNA markers, M) was detected using a PhosphorImager. An excess of competitor DNA was added to remove FACT from FACT-nucleosome complexes.
b,c, Typical frequency distributions of proximity ratios (EPR) for nucleosomes N35/112 in the presence/absence of Nhp6, Spt16/Pob3 or FACT with/without competitor DNA. Analysis by spFRET microscopy. The sample sizes (n, single particle events) were the following: (N) – 4622; (N+Nhp6) – 14128; (N+Spt16/Pob3) – 15779; (N+FACT) – 5958; (N+FACT + competitor DNA) – 9297. The mean values of EPR peaks and the standard errors were the following: (N) – 0.02±0.03, 0.653±0.004; (N+Nhp6) – 0.03±0.03, 0.620±0.004; (N+Spt16/Pob3) – 0.01±0.07, 0.64±0.01; (N+FACT) – 0.033±0.003, 0.51±0.03; (N+FACT+ competitor DNA) – 0.02±0.03, 0.65±0.06. b, Addition of Nhp6 or Spt16/Pob3 to the nucleosomes induced minor changes in EPR of the majority of nucleosomes that indicates minimal changes in the folding of nucleosomal DNA near the contact of H2A-H2B dimers.
c, FACT induces unfolding of the nucleosomal DNA which can be reversed by addition of an excess of competitor DNA disrupting the FACT:nucleosome complexes.