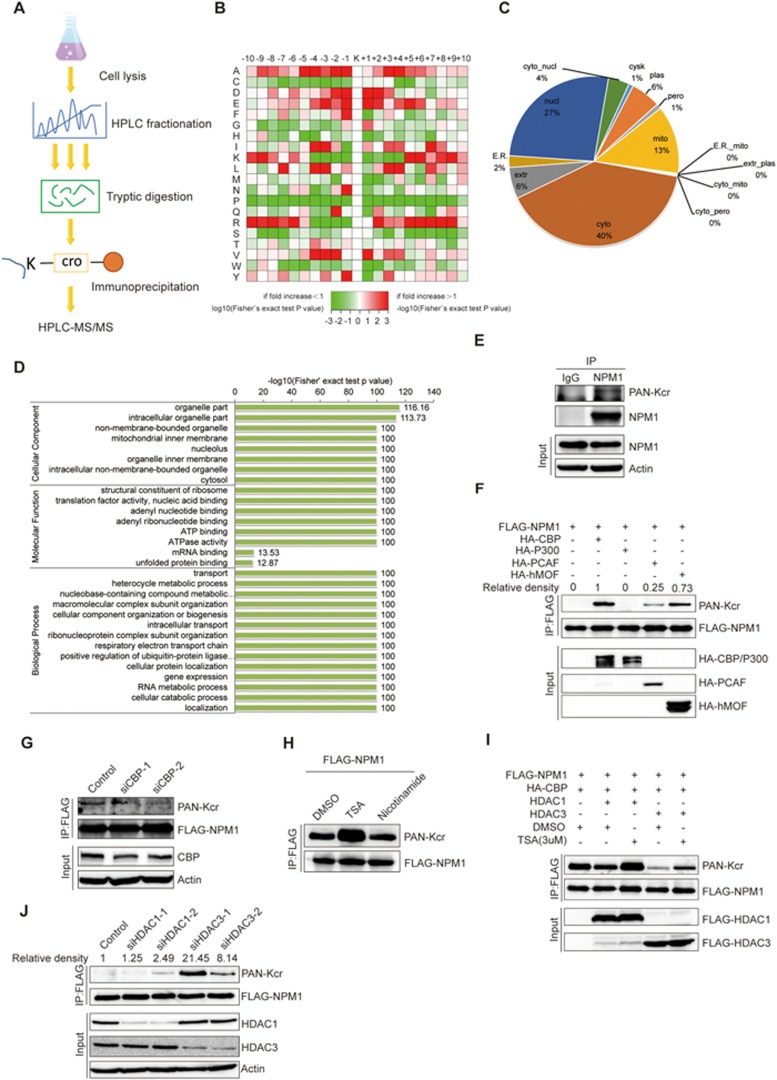
Figure 1.
Identification of crotonylation on non-histone proteins. (A) Flowchart illustrating the experimental procedure for identification of crotonylated proteins by proteomics. (B) Motif analysis of all identified crotonylated sites. (C) Subcellular localization of the proteins with lysine crotonylation. (D) GO-based enrichment analysis of crotonylated proteins. (E) Validation of endogenous crotonylated NPM1. H1299 cells were treated with 3 μM TSA and 5 mM nicotinamide for 12 h, and cell lysates were immunoprecipitated with an anti-NPM1 antibody, followed by immunoblotting with a pan-Kcr antibody. (F) FLAG-tagged NPM1 was cotransfected into H1299 cells with a variety of acetyltransferases. Forty-eight hours post transfection, cell lysates were immunoprecipitated with an anti-FLAG antibody, followed by western blot analysis with a pan-Kcr antibody. (G) H1299 cells were transfected with FLAG-NPM1, and endogenous CBP was knocked down with siRNA. Cells were treated with 3 μM TSA for 12 h, and cell lysates were immunoprecipitated with an anti-FLAG antibody, followed by western blot analysis with a pan-Kcr antibody. (H) The HDAC inhibitor TSA increased the crotonylation level of NPM1. H1299 cells were transfected with FLAG-NPM1 and HA-CBP, and then treated with 3 μM TSA or 5 mM nicotinamide for 12 h. Cell lysates were immunoprecipitated with an anti-FLAG antibody, followed by western blot analysis with a pan-Kcr antibody. (I) H1299 cells were co-transfected with FLAG-NPM1, HA-CBP, and FLAG-HDAC1 or FLAG-HDAC3, and then treated with 3 μM TSA for 12 h. Western blot analysis was performed with a pan-Kcr antibody. (J) H1299 cells were co-transfected with FLAG-NPM1 and HA-CBP. Endogenous HDAC1 or HDAC3 was knocked down with siRNAs. Cell lysates were immunoprecipitated with an anti-FLAG antibody, followed by western blot analysis with a pan-Kcr antibody.