Abstract
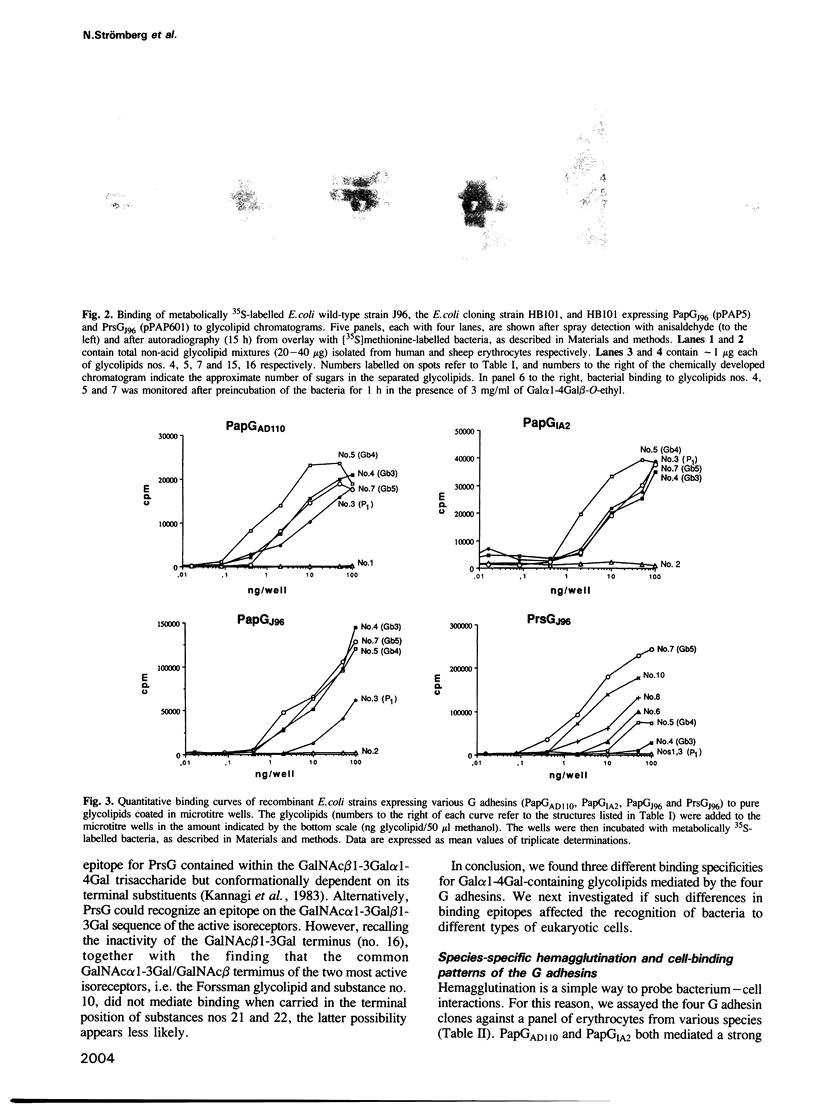
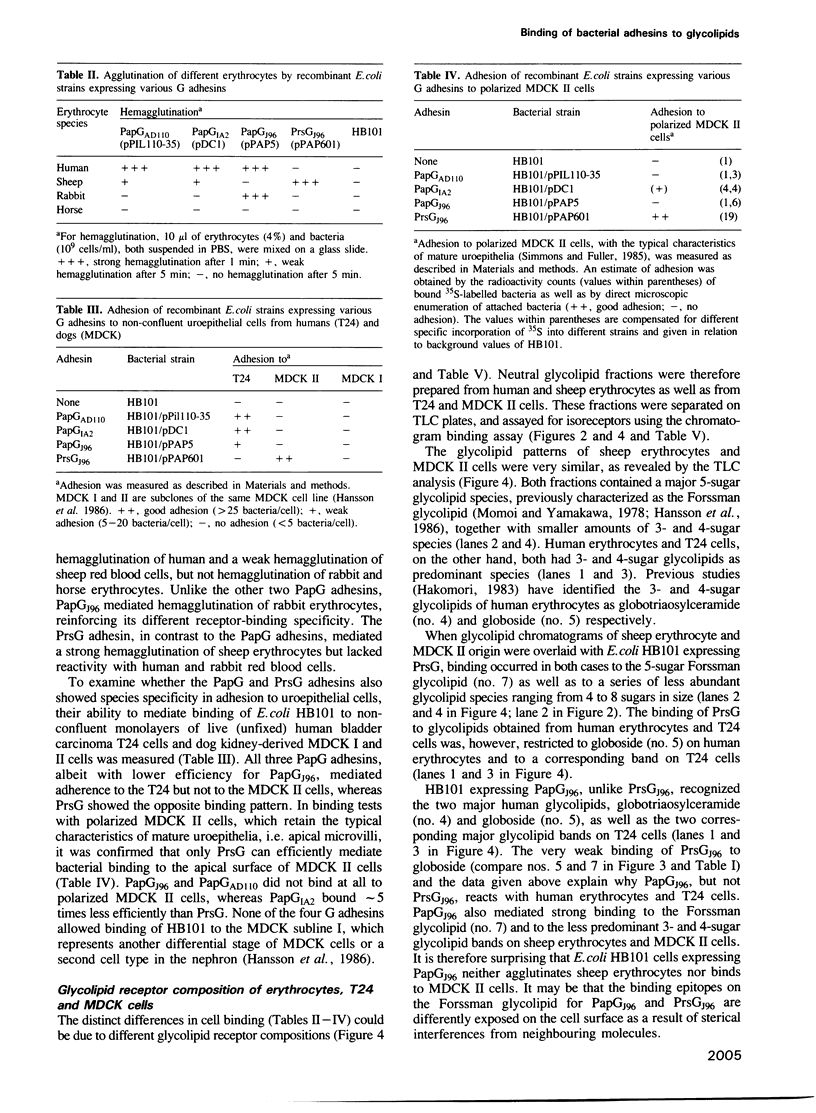
Four G adhesins, cloned from uropathogenic Escherichia coli strains, were examined for binding to glycolipids and various eukaryotic cells. PapGAD110 and PapGIA2 showed virtually identical binding patterns to Gal alpha 1-4Gal-containing glycolipids, while PapGJ96 differed slightly and PrsGJ96 markedly with respect to the effect of neighbouring groups on the binding. Their hemagglutination patterns confirmed the existence of three receptor-binding specificities. While the PapG adhesins bound to uroepithelial cells from man (T24) but not to those from the dog (MDCK II), the reverse was true of PrsG. These binding patterns were largely explained by the absence or presence of appropriate glycolipid isoreceptors, although the inability of the PapG adhesins to bind MDCK II cells was attributed to an inappropriate presentation of their receptor epitopes. The high prevalence of PrsG-like specificities observed among wild-type dog uropathogenic E. coli isolates, together with the determined isoreceptor composition of human and dog kidney target tissues, suggest variation in receptor specificity as a mechanism for shifting host specificity, and that this variation has evolved in response to the topography of the host cellular receptors. The receptor-binding half proposed for the predicted amino acid sequences of the four G adhesins and the corresponding adhesin of one of the dog E. coli isolates varied considerably among the three receptor-binding groups of adhesins, but only little within each group.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angström J., Karlsson H., Karlsson K. A., Larson G., Nilson K. GalNAc beta 1----3 terminated glycosphingolipids of human erythrocytes. Arch Biochem Biophys. 1986 Dec;251(2):440–449. doi: 10.1016/0003-9861(86)90350-4. [DOI] [PubMed] [Google Scholar]
- Blomberg J., Breimer M. E., Karlsson K. A. Glycosphingolipids of a green monkey kidney cell line (GMK AH-1). Evidence for a novel pentaglycosylceramide based on globotetraosylceramide. Biochim Biophys Acta. 1982 Jun 11;711(3):466–477. doi: 10.1016/0005-2760(82)90061-3. [DOI] [PubMed] [Google Scholar]
- Bock K., Breimer M. E., Brignole A., Hansson G. C., Karlsson K. A., Larson G., Leffler H., Samuelsson B. E., Strömberg N., Edén C. S. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1----4Gal-containing glycosphingolipids. J Biol Chem. 1985 Jul 15;260(14):8545–8551. [PubMed] [Google Scholar]
- Breimer M. E., Hansson G. C., Leffler H. The specific glycosphingolipid composition of human ureteral epithelial cells. J Biochem. 1985 Nov;98(5):1169–1180. doi: 10.1093/oxfordjournals.jbchem.a135383. [DOI] [PubMed] [Google Scholar]
- Breimer M. E., Jovall P. A. Structural characterization of a blood group A heptaglycosylceramide with globo-series structure. The major glycolipid based blood group A antigen of human kidney. FEBS Lett. 1985 Jan 1;179(1):165–172. doi: 10.1016/0014-5793(85)80213-1. [DOI] [PubMed] [Google Scholar]
- Breimer M. E., Karlsson K. A. Chemical and immunological identification of glycolipid-based blood group ABH and Lewis antigens in human kidney. Biochim Biophys Acta. 1983 Jan 25;755(2):170–177. doi: 10.1016/0304-4165(83)90200-3. [DOI] [PubMed] [Google Scholar]
- Bubeník J., Baresová M., Viklický V., Jakoubková J., Sainerová H., Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer. 1973 May;11(3):765–773. doi: 10.1002/ijc.2910110327. [DOI] [PubMed] [Google Scholar]
- Clegg S. Cloning of genes determining the production of mannose-resistant fimbriae in a uropathogenic strain of Escherichia coli belonging to serogroup O6. Infect Immun. 1982 Nov;38(2):739–744. doi: 10.1128/iai.38.2.739-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto T., Ichikawa Y., Nishimura K., Ando S., Yamakawa T. Chemistry of lipid of the posthemyolytic residue or stroma of erythrocytes. XVI. Occurrence of ceramide pentasaccharide in the membrane of erythrocytes and reticulocytes of rabbit. J Biochem. 1968 Aug;64(2):205–213. doi: 10.1093/oxfordjournals.jbchem.a128881. [DOI] [PubMed] [Google Scholar]
- Falk P., Holgersson J., Jovall P. A., Karlsson K. A., Strömberg N., Thurin J., Brodin T., Sjögren H. O. An antigen present in rat adenocarcinoma and normal colon non-epithelial stroma is a novel Forssman-like glycolipid based on isoglobotetraosylceramide. Biochim Biophys Acta. 1986 Sep 12;878(2):296–299. doi: 10.1016/0005-2760(86)90161-x. [DOI] [PubMed] [Google Scholar]
- Fewster M. E., Burns B. J., Mead J. F. Quantitative densitometric thin-layer chromatography of lipids using copper acetate reagent. J Chromatogr. 1969 Aug 5;43(1):120–126. doi: 10.1016/s0021-9673(00)99173-8. [DOI] [PubMed] [Google Scholar]
- Garcia E., Bergmans H. E., Van den Bosch J. F., Orskov I., Van der Zeijst B. A., Gaastra W. Isolation and characterisation of dog uropathogenic Escherichia coli strains and their fimbriae. Antonie Van Leeuwenhoek. 1988;54(2):149–163. doi: 10.1007/BF00419202. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., McKibbin J. M., Strömberg N., Thurin J. Isoglobotriaosylceramide and the Forssman glycolipid of dog small intestine occupy separate tissue compartments and differ in ceramide composition. Biochim Biophys Acta. 1983 Jan 7;750(1):214–216. doi: 10.1016/0005-2760(83)90224-2. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., Strömberg N., Thurin J. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: a rationalized approach to the study of host cell glycolipid receptors. Anal Biochem. 1985 Apr;146(1):158–163. doi: 10.1016/0003-2697(85)90410-5. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Leffler H., Strömberg N. Gangliotetraosylceramide is a major glycolipid of epithelial cells of mouse small intestine. FEBS Lett. 1982 Mar 22;139(2):291–294. doi: 10.1016/0014-5793(82)80873-9. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Simons K., van Meer G. Two strains of the Madin-Darby canine kidney (MDCK) cell line have distinct glycosphingolipid compositions. EMBO J. 1986 Mar;5(3):483–489. doi: 10.1002/j.1460-2075.1986.tb04237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R., Levery S. B., Ishigami F., Hakomori S., Shevinsky L. H., Knowles B. B., Solter D. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J Biol Chem. 1983 Jul 25;258(14):8934–8942. [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Larson G. Molecular characterization of cell surface antigens of fetal tissue. Detailed analysis of glycosphingolipids of meconium of a human O Le(a--b+) secretor. J Biol Chem. 1981 Apr 10;256(7):3512–3524. [PubMed] [Google Scholar]
- Karlsson K. A., Larson G. Structural characterization of lactotetraosylceramide, a novel glycosphingolipid isolated from human meconium. J Biol Chem. 1979 Sep 25;254(18):9311–9316. [PubMed] [Google Scholar]
- Karlsson K. A. Preparation of total nonacid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 1987;138:212–220. doi: 10.1016/0076-6879(87)38018-8. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen V., Saxén H., Hultberg H., Svenson S. B. P-antigen-recognizing fimbriae from human uropathogenic Escherichia coli strains. Infect Immun. 1982 Jul;37(1):286–291. doi: 10.1128/iai.37.1.286-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S. K., Samuelsson B. E., Pascher I., Marcus D. M. New gangliosides from human erythrocytes. J Biol Chem. 1983 Nov 25;258(22):13857–13866. [PubMed] [Google Scholar]
- Lampio A., Rauvala H., Gahmberg C. G. Exposure of major neutral glycolipids in red cells to galactose oxidase. Effect of neuraminidase. Eur J Biochem. 1986 Jun 16;157(3):611–616. doi: 10.1111/j.1432-1033.1986.tb09709.x. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Brown J. E., Strömberg N., Westling-Ryd M., Schultz J. E., Karlsson K. A. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987 Feb 5;262(4):1779–1785. [PubMed] [Google Scholar]
- Lindberg F. P., Lund B., Normark S. Genes of pyelonephritogenic E. coli required for digalactoside-specific agglutination of human cells. EMBO J. 1984 May;3(5):1167–1173. doi: 10.1002/j.1460-2075.1984.tb01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Lund B., Lindberg F. P., Båga M., Normark S. Globoside-specific adhesins of uropathogenic Escherichia coli are encoded by similar trans-complementable gene clusters. J Bacteriol. 1985 Jun;162(3):1293–1301. doi: 10.1128/jb.162.3.1293-1301.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Marklund B. I., Strömberg N., Lindberg F., Karlsson K. A., Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988 Mar;2(2):255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- McKibbin J. M., Spencer W. A., Smith E. L., Mansson J. E., Karlsson K. A., Samuelsson B. E., Li Y. T., Li S. C. Lewis blood group fucolipids and their isomers from human and canine intestine. J Biol Chem. 1982 Jan 25;257(2):755–760. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Momoi M., Yamakawa T. Glucosamine-containing sphingoglycolipids from sheep erythrocytes. J Biochem. 1978 Aug;84(2):317–325. doi: 10.1093/oxfordjournals.jbchem.a132131. [DOI] [PubMed] [Google Scholar]
- Naiki M., Fong J., Ledeen R., Marcus D. M. Structure of the human erythrocyte blood group P1 glycosphingolipid. Biochemistry. 1975 Nov 4;14(22):4831–4837. doi: 10.1021/bi00693a009. [DOI] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Lark D., Hull R., Norgren M., Båga M., O'Hanley P., Schoolnik G., Falkow S. Genetics of digalactoside-binding adhesin from a uropathogenic Escherichia coli strain. Infect Immun. 1983 Sep;41(3):942–949. doi: 10.1128/iai.41.3.942-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascher I., Sundell S., Harlos K., Eibl H. Conformation and packing properties of membrane lipids: the crystal structure of sodium dimyristoylphosphatidylglycerol. Biochim Biophys Acta. 1987 Jan 9;896(1):77–88. doi: 10.1016/0005-2736(87)90358-0. [DOI] [PubMed] [Google Scholar]
- Pritchett T. J., Brossmer R., Rose U., Paulson J. C. Recognition of monovalent sialosides by influenza virus H3 hemagglutinin. Virology. 1987 Oct;160(2):502–506. doi: 10.1016/0042-6822(87)90026-2. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C., Daniels R. S., Skehel J. J., Wilson I. A., Wiley D. C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983 Jul 7;304(5921):76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983 Jun;127(2):361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior D., Baker N., Cedergren B., Falk P., Larson G., Lindstedt R., Edén C. S. Globo-A--a new receptor specificity for attaching Escherichia coli. FEBS Lett. 1988 Sep 12;237(1-2):123–127. doi: 10.1016/0014-5793(88)80184-4. [DOI] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Stromberg N., Deal C., Nyberg G., Normark S., So M., Karlsson K. A. Identification of carbohydrate structures that are possible receptors for Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4902–4906. doi: 10.1073/pnas.85.13.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg N., Ryd M., Lindberg A. A., Karlsson K. A. Studies on the binding of bacteria to glycolipids. Two species of Propionibacterium apparently recognize separate epitopes on lactose of lactosylceramide. FEBS Lett. 1988 May 9;232(1):193–198. doi: 10.1016/0014-5793(88)80415-0. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Esselman W. J., Sweeley C. C. Structure of a pentahexosylceramide (Forssman hapten) from canine intestine and kidney. J Biol Chem. 1973 Sep 25;248(18):6528–6533. [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988 Jun 2;333(6172):426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]